Thiotepa for Injection, USP
Thiotepa by
Drug Labeling and Warnings
Thiotepa by is a Prescription medication manufactured, distributed, or labeled by STI Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
THIOTEPA- thiotepa injection, powder, lyophilized, for solution
STI Pharma LLC
----------
Thiotepa for Injection, USP
DESCRIPTION
Thiotepa for Injection, USP is an ethylenimine-type compound. It is supplied as a non-pyrogenic, sterile Iyophilized powder for intravenous, intracavitary or intravesical administration, containing 15 mg of thiotepa. Thiotepa is a synthetic product with antitumor activity. The chemical name for thiotepa is
Tris(1-aziridinyl)phosphine sulfide. Thiotepa has the following structural formula:
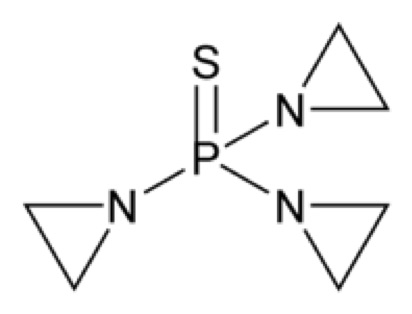
Thiotepa has the molecular formula C 6H 12N 3PS, and a molecular weight of 189.22. When reconstituted with sterile water for injection, the resulting solution has a pH of approximately 5.5 to 7.5. Thiotepa is stable in alkaline medium and unstable in acid medium.
CLINICAL PHARMACOLOGY
Thiotepa is a cytotoxic agent of the polyfunctional type, related chemically and pharmacologically to nitrogen mustard. The radiomimetic action of thiotepa is believed to occur through the release of ethylenimine radicals which, like irradiation, disrupt the bonds of DNA. One of the principal bond disruptions is initiated by alkylation of guanine at the N-7 position, which severs the linkage between the purine base and the sugar and liberates alkylated guanines.
The pharmacokinetics of thiotepa and TEPA in thirteen female patients (45 to 84 years) with advanced stage ovarian cancer receiving 60 mg and 80 mg thiotepa by intravenous infusion on subsequent courses given at 4-week intervals are presented in the following table:
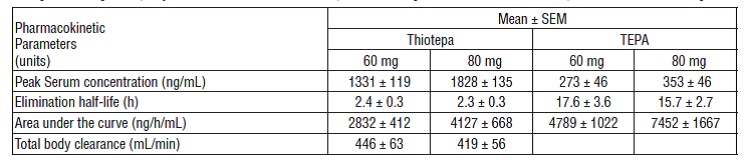
TEPA, which possesses cytotoxic activity, appears to be the major metabolite of thiotepa found in human serum and urine. Urinary excretion of 14C-labeled thiotepa and metabolites in a 34 year old patient with metastatic carcinoma of the cecum who received a dose of 0.3 mg/kg intravenously was 63%. Thiotepa and TEPA in urine each accounts for less than 2% of the administered dose.
The pharmacokinetics of thiotepa in renal and hepatic dysfunction patients have not been evaluated. Possible pharmacokinetic interactions of thiotepa with any concomitantly administered medications have not been formally investigated.
INDICATIONS AND USAGE
Thiotepa for Injection, USP has been tried with varying results in the palliation of a wide variety of neoplastic diseases. However, the most consistent results have been seen in the following tumors:
1. Adenocarcinoma of the breast.
2. Adenocarcinoma of the ovary.
3. For controlling intracavitary effusions secondary to diffuse or localized neoplastic diseases of various serosal cavities.
4. For the treatment of superficial papillary carcinoma of the urinary bladder.
While now largely superseded by other treatments, thiotepa has been effective against other lymphomas, such as lymphosarcoma and Hodgkin's disease.
CONTRAINDICATIONS
Thiotepa is contraindicated in patients with a known hypersensitivity (allergy) to this preparation.
Therapy is probably contraindicated in cases of existing hepatic, renal, or bone-marrow damage. However, if the need outweighs the risk in such patients, thiotepa may be used in low dosage, and accompanied by hepatic, renal and hemopoietic function tests.
WARNINGS
Death has occurred after intravesical administration, caused by bone-marrow depression from systematically absorbed drug.
Death from septicemia and hemorrhage has occurred as a direct result of hematopoietic depression by thiotepa.
Thiotepa is highly toxic to the hematopoietic system. A rapidly falling white blood cell or platelet count indicates the necessity for discontinuing or reducing the dosage of thiotepa. Weekly blood and platelet counts are recommended during therapy and for at least 3 weeks after therapy has been discontinued.
Thiotepa can cause fetal harm when administered to a pregnant woman. Thiotepa given by the intraperitoneal (IP) route was teratogenic in mice at doses ≥ 1 mg/kg (3.2 mg/m 2), approximately 8-fold less than the maximum recommended human therapeutic dose (0.8 mg/kg, 27 mg/m 2), based on body-surface area. Thiotepa given by the IP route was teratogenic in rats at doses ≥ 3 mg/kg (21 mg/m 2), approximately equal to the maximum recommended human therapeutic dose, based on body-surface area. Thiotepa was lethal to rabbit fetuses at a dose of 3 mg/kg (41 mg/m 2), approximately two times the maximum recommended human therapeutic dose based on body-surface area.
Effective contraception should be used during thiotepa therapy if either the patient or partner is of childbearing potential. There are no adequate and well-controlled studies in pregnant women. If thiotepa is used during pregnancy, or if pregnancy occurs during thiotepa therapy, the patient and partner should be apprised of the potential hazard to the fetus.
Thiotepa is a polyfunctional alkylating agent, capable of cross-linking the DNA within a cell and changing its nature. The replication of the cell is, therefore, altered, and thiotepa may be described as mutagenic. An in vitro study has shown that it causes chromosomal aberrations of the chromatid type and that the frequency of induced aberrations increases with the age of the subject.
Like many alkylating agents, thiotepa has been reported to be carcinogenic when administered to laboratory animals. Carcinogenicity is shown most clearly in studies using mice, but there is some evidence of carcinogenicity in man. In patients treated with thiotepa, cases of myelodysplastic syndromes and acute non-lymphocytic leukemia have been reported.
PRECAUTIONS
General
The serious complication of excessive thiotepa therapy, or sensitivity to the effects of thiotepa, is bone-marrow depression. If proper precautions are not observed thiotepa may cause leukopenia, thrombocytopenia, and anemia.
Information for Patients
The patient should notify the physician in the case of any sign of bleeding (epistaxis, easy bruising, change in color of urine, black stool) or infection (fever, chills) or for possible pregnancy to patient or partner.
Effective contraception should be used during thiotepa therapy if either the patient or the partner is of childbearing potential.
Laboratory Tests
The most reliable guide to thiotepa toxicity is the white blood cell count. If this falls to 3000 or less, the dose should be discontinued. Another good index of thiotepa toxicity is the platelet count; if this falls to 150,000, therapy should be discontinued. Red blood cell count is a less accurate indicator of thiotepa toxicity. If the drug is used in patients with hepatic or renal damage (see CONTRAINDICATIONS section), regular assessment of hepatic and renal function tests are indicated.
Drug Interactions
It is not advisable to combine, simultaneously or sequentially, cancer chemotherapeutic agents or a cancer chemotherapeutic agent and a therapeutic modality having the same mechanism of action. Therefore, thiotepa combined with other alkylating agents such as nitrogen mustard or cyclophosphamide or thiotepa combined with irradiation would serve to intensify toxicity rather than to enhance therapeutic response. If these agents must follow each other, it is important that recovery from the first agent, as indicated by white blood cell count, be complete before therapy with the second agent is instituted.
Other drugs which are known to produce bone-marrow depression should be avoided.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Also see WARNINGS section.
Carcinogenesis
In mice, repeated IP administration of thiotepa (1.15 or 2.3 mg/kg three times per week for 52 or 43 weeks, respectively) produced a significant increase in the combined incidence of squamous-cell carcinomas of the skin, preputial gland, and ear canal, and combined incidence of lymphoma and lymphocytic leukemia. In other studies in mice, repeated IP administration of thiotepa (4 or 8 mg/kg three times per week for 4 weeks followed by a 20 week observation period or 1.8 mg/kg three times per week for 4 weeks followed by a 35 week observation period) resulted in an increased incidence of lung tumors. In rats, repeated IP administration of thiotepa (0.7 or 1.4 mg/kg three times per week for 52 or 34 weeks, respectively) produced significant increases in the incidence of squamous-cell carcinomas of the skin or ear canal, combined hematopoietic neoplasms, and uterine adenocarcinomas. Thiotepa given intravenously (IV) to rats (1 mg/kg once per week for 52 weeks) produced an increased incidence of malignant tumors (abdominal cavity sarcoma, lymphosarcoma myelosis, seminoma, fibrosarcoma, salivary gland hemangioendothelioma, mammary sarcoma, pheochromocytoma) and benign tumors.
The lowest reported carcinogenic dose in mice (1.15 mg/kg, 3.68 mg/m
2) is approximately 7-fold less than the maximum recommended human therapeutic dose based on body- surface area. The lowest reported carcinogenic dose in rats (0.7 mg/kg, 4.9 mg/m
2) is approximately 6-fold less than the maximum recommended human therapeutic dose based on body-surface area.
Mutagenesis
Thiotepa was mutagenic in in vitro assays in Salmonella typhimurium, E coli, Chinese hamster lung and human lymphocytes. Chromosomal aberrations and sister chromatid exchanges were observed in vitro with thiotepa in bean root tips, human lymphocytes, Chinese hamster lung, and monkey lymphocytes. Mutations were observed with oral thiotepa in mouse at doses > 2.5 mg/kg (8 mg/m 2). The mouse micronucleus test was positive with IP administration of > 1 mg/kg (3.2 mg/m 2). Other positive in vivo chromosomal aberration or mutation assays included Drosophila melanogaster, Chinese hamster marrow, murine marrow, monkey lymphocyte, and murine germ cell.
Impairment of Fertility
Thiotepa impaired fertility in male mice at PO or IP doses ≥ 0.7 mg/kg (2.24 mg/m 2), approximately 12-fold less than the maximum recommended human therapeutic dose based on body-surface area. Thiotepa (0.5 mg) inhibited implantation in female rats when instilled into the uterine cavity. Thiotepa interfered with spermatogenesis in mice at IP doses ≥ 0.5 mg/kg (1.6 mg/m 2), approximately 17-fold less than the maximum recommended human therapeutic dose based on body-surface area. Thiotepa interfered with spermatogenesis in hamsters at an IP dose of 1 mg/kg (4.1 mg/m 2), approximately 7-fold less than the maximum recommended human therapeutic dose based on body-surface area.
Pregnancy
Teratogenic Effects - Category D
See WARNINGS section.
Thiotepa can cause fetal harm when administered to a pregnant woman. Thiotepa given by the IP route was teratogenic in mice at doses ≥ 1 mg/kg (3.2 mg/m 2), approximately 8-fold less than the maximum recommended human therapeutic dose based on body-surface area. Thiotepa given by the IP route was teratogenic in rats at doses ≥3 mg/kg (21 mg/m 2), approximately equal to the maximum recommended human therapeutic dose based on body-surface area. Thiotepa was lethal to rabbit fetuses at a dose of 3 mg/kg (41 mg/m 2), approximately 2 times the maximum recommended human therapeutic dose based on body-surface area. Patients of childbearing potential should be advised to avoid pregnancy. There are no adequate and well-controlled studies in pregnant women. If thiotepa is used during pregnancy, or if pregnancy occurs during thiotepa therapy, the patient and partner should be apprised of the potential hazard to the fetus.
Nursing Mothers
It is not known whether thiotepa is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for tumorigenicity shown for thiotepa in animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Clinical studies of thiotepa did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreasing hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
In addition to its effect on the blood-forming elements (see WARNINGSand PRECAUTIONS sections), thiotepa may cause other adverse reactions.
General
Fatigue, weakness. Febrile reaction and discharge from a subcutaneous lesion may occur as the result of breakdown of tumor tissue.
Hypersensitivity Reactions
Allergic reactions - rash, urticaria, laryngeal edema, asthma, anaphylactic shock, wheezing.
Renal
Dysuria, urinary retention. There have been rare reports of chemical cystitis or hemorrhagic cystitis following intravesical, but not parenteral administration of thiotepa.
OVERDOSAGE
Hematopoietic toxicity can occur following overdose, manifested by a decrease in the white cell count and/or platelets. Red blood cell count is a less accurate indicator of thiotepa toxicity. Bleeding manifestations may develop. The patient may become more vulnerable to infection, and less able to combat such infection.
Dosages within and minimally above the recommended therapeutic doses have been associated with potentially life-threatening hematopoietic toxicity. Thiotepa has a toxic effect on the hematopoietic system that is dose related.
Thiotepa is dialyzable.
There is no known antidote for overdosage with thiotepa. Transfusions of whole blood or platelets have proven beneficial to the patient in combating hematopoietic toxicity.
DOSAGE AND ADMINISTRATION
Since absorption from the gastrointestinal tract is variable, thiotepa should not be administered orally.
Dosage must be carefully individualized. A slow response to thiotepa does not necessarily indicate a lack of effect. Therefore, increasing the frequency of dosing may only increase toxicity. After maximum benefit is obtained by initial therapy, it is necessary to continue the patient on maintenance therapy (1 to 4 week intervals). In order to continue optimal effect, maintenance doses should not be administered more frequently than weekly in order to preserve correlation between dose and blood counts.
Preparation and Administration Precautions
Thiotepa is a cytotoxic anticancer drug and as with other potentially toxic compounds, caution should be exercised in handling and preparation of thiotepa. Skin reactions associated with accidental exposure to thiotepa may occur. The use of gloves is recommended. If thiotepa solution contacts the skin, immediately wash the skin thoroughly with soap and water. If thiotepa contacts mucous membranes, the membranes should be flushed thoroughly with water.
Preparation of Solution
Thiotepa for injection should be reconstituted with 1.5 mL of sterile water for injection resulting in a drug concentration of approximately 10 mg/mL. The actual withdrawable quantities and concentration achieved are illustrated in the following table:
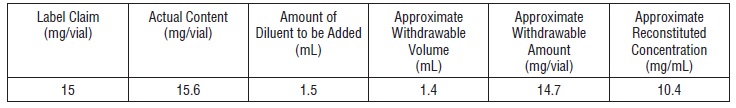
The reconstituted solution is hypotonic and should be further diluted with sodium chloride injection (0. 9% sodium chloride) before use.
When reconstituted with sterile water for injection, solutions of thiotepa should be stored in a refrigerator and used within 8 hours. Reconstituted solutions further diluted with sodium chloride injection should be used immediately.
In order to eliminate haze, filter solutions through a 0.22 micron filter* prior to administration. Filtering does not alter solution potency. Reconstituted solutions should be clear. Solutions that remain opaque or precipitate after filtration should not be used.
*Polysulfone membrane (Gelman’s Sterile Aerodisc®, Single Use) or triton-free mixed ester of cellulose/PVC (Millipore’s MILLEX®-GS Filter Unit).
Initial and Maintenance Doses
Initially the higher dose in the given range is commonly administered. The maintenance dose should be adjusted weekly on the basis of pretreatment control blood counts andsubsequent blood counts.
Intravenous Administration
Thiotepa may be given by rapid intravenous administration in doses of 0.3 to 0.4 mg/kg. Doses should be given at 1 to 4 week intervals.
Intracavitary Administration
The dosage recommended is 0.6 to 0.8 mg/kg. Administration is usually effected through the same tubing which is used to remove the fluid from the cavity involved.
Intravesical Administration
Patients with papillary carcinoma of the bladder are dehydrated for 8 to 12 hours prior to treatment. Then 60 mg of thiotepa in 30 to 60 mL of Sodium Chloride Injection is instilled into the bladder by catheter. For maximum effect, the solution should be retained for 2 hours. If the patient finds it impossible to retain 60 mL for 2 hours, the dose may be given in a volume of 30 mL. If desired, the patient may be positioned every 15 minutes for maximum area contact. The usual course of treatment is once a week for 4 weeks. The course may be repeated if necessary, but second and third courses must be given with caution since bone-marrow depression may be increased. Deaths have occurred after intravesical administration, caused by bone-marrow depression from systemically absorbed drug.
Handling and Disposal
Follow safe cytotoxic agent handling procedures. Several guidelines on this subject have been published. 1-7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
Thiotepa for Injection, USP, for single use only, is available in vials containing 15 mg of nonpyrogenic, sterile lyophilized powder, supplied as follows:
NDC: 54879-014-13. Unit carton contains 1 x 15 mg single dose vial thiotepa.
Store in a refrigerator between 2° to 8°C (36° to 46°F).
PROTECT FROM LIGHT AT ALL TIMES.
REFERENCES
1. Recommendations for the Safe Handling of Parenteral Antineoplastic Drugs. NIH Publication No. 83-2621. For sale by the Superintendent of Documents, US Government Printing Office, Washington, DC 20402.
2. AMA Council Report. Guidelines for Handling Parenteral Antineoplastics. JAMA. 1985;253(11):1590-1592.
3. National Study Commission on Cytotosic Exposure - Recommendations for Handling Cytotoxic Agents. Available from Louis P. Jeffrey, Sc D, Chairman, National Study Commission on Cytotoxic Exposure, Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, Massachusetts 02115.
4. Clinical Oncological Society of Australia: Guidelines and recommendations for safe handling of antineoplastic agents. Med J Australia. 1983; 1:426-428.
5. Jones RB, et al. Safe handling of chemotherapeutic agents: A report from the Mount Sinai Medical Center. Ca - A Cancer Journal for Clinicians. Sept/Oct 1983; 258-263.
6. American Society of Hospital Pharmacists technical assistance bulletin on handling cytotoxic and hazardous drugs. Am J Hosp Pharm. 1990; 47:1033-1049.
7. Controlling Occupational Exposure to Hazardous Drugs. (OSHA WORK-PRACTICE GUIDELINES). AM J Health-Syst Pharm. 1996:53:1669-1685.
| THIOTEPA
thiotepa injection, powder, lyophilized, for solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - STI Pharma LLC (832714070) |
| Registrant - STI Pharma LLC (832714070) |
Trademark Results [Thiotepa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 THIOTEPA 73471323 not registered Dead/Abandoned |
AMERICAN CYANAMID COMPANY 1984-03-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
