REDEMPLO- plozasiran injection, solution
REDEMPLO by
Drug Labeling and Warnings
REDEMPLO by is a Prescription medication manufactured, distributed, or labeled by Arrowhead Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REDEMPLO® safely and effectively. See full prescribing information for REDEMPLO.
REDEMPLO (plozasiran) injection, for subcutaneous use
Initial U.S. Approval: 2025INDICATIONS AND USAGE
REDEMPLO is an apolipoprotein C-III (apoC-III)-directed small interfering ribonucleic acid (siRNA) indicated as an adjunct to diet to reduce triglycerides in adults with familial chylomicronemia syndrome (FCS). (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage of REDEMPLO is 25 mg injected subcutaneously once every 3 months. (2.1)
- Inject REDEMPLO subcutaneously into the front of the thigh or abdomen. The outer area of the upper arm can be used as an injection site if a healthcare provider or caregiver administers the injection. (2.2)
DOSAGE FORMS AND STRENGTHS
Injection: 25 mg/0.5 mL solution in a single-dose pre-filled syringe. (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
Most common adverse reactions in REDEMPLO treated patients (incidence ≥10% of patients treated with REDEMPLO and >5% more frequently than with placebo) are hyperglycemia, headache, nausea, and injection site reaction. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Arrowhead Pharmaceuticals Inc. at 1-844-REDEMPLO (1-844-733-3675), or https://arrowheadpharma.com/safetyreporting, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
- The recommended dosage of REDEMPLO is 25 mg injected subcutaneously once every 3 months.
2.2 Important Administration Instructions
- Prior to initiation, train patients and/or caregivers on proper preparation and administration of REDEMPLO [see Instructions for Use].
- Adhere to a low-fat diet (less than or equal to 20 grams fat per day) in conjunction with REDEMPLO.
- Visually inspect the REDEMPLO pre-filled syringe prior to administration. The solution should be clear and colorless to yellow. Do not use if cloudiness, particulate matter, or discoloration is observed prior to administration.
- Inject REDEMPLO subcutaneously into the front of the thigh or abdomen. The outer area of the upper arm can be used as an injection site if a healthcare provider or caregiver administers the injection.
- Do not inject REDEMPLO in an area where the skin is damaged (tender, bruised, red, hard, or cut). Do not inject into areas with scars or stretch marks.
- If a dose is missed, administer REDEMPLO as soon as possible. Resume dosing every 3 months from the date of the most recently administered dose.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of REDEMPLO cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of REDEMPLO was evaluated in 75 patients with FCS enrolled in Trial 1 (NCT05089084) [see Clinical Studies (14)]. In this trial, patients received at least one dose of REDEMPLO 25 mg (N=26) or 50 mg of plozasiran (N=24) and 25 patients received placebo. Plozasiran 50 mg is not an approved dosage regimen for FCS [see Dosage and Administration (2.1)]. Across treatment groups, the mean age was 46 years and 49% of patients were male. Seventy-three percent (73%) of patients were White, 21% were Asian, and 5% were reported as other races; 3% identified as Hispanic or Latino ethnicity. Fifty (50) patients were exposed to REDEMPLO for a median of 11.6 months; 26 patients were treated with REDEMPLO 25 mg every 3 months for a median of 11.8 months.
Adverse reactions led to discontinuation of treatment in 3 (6.0%) of REDEMPLO-treated patients and 0% of placebo-treated patients. The reasons for REDEMPLO treatment discontinuation were hyperglycemia and urticaria. Adverse reactions occurring in greater than or equal to 10% of REDEMPLO-treated patients and greater than 5% more frequently than in placebo-treated patients are listed below in Table 1.
Table 1. Adverse Reactions Occurring in Greater than or Equal to 10% of REDEMPLO-treated Patients and Greater than 5% More Frequently than with Placebo in Trial 1 1Grouped terms composed of several similar terms
Adverse Reactions Placebo
(N=25) (%)REDEMPLO
(N=50) (%)Hyperglycemia1 2 (8%) 10 (20%) Headache 2 (8%) 8 (16%) Nausea 2 (8%) 7 (14%) Injection site reaction1 1 (4%) 5 (10%) Laboratory Tests
Increase in Glucose: Mean increases from baseline in HbA1c (up to 0.36%) and fasting glucose (up to 9 mg/dL) were observed over time in the 25 mg REDEMPLO group. The incidence of hyperglycemia (defined as adverse events consistent with diabetes mellitus or hyperglycemia, new antidiabetic medication, or laboratory values) was higher in 25 mg REDEMPLO-treated patients without a medical history of diabetes at baseline (40%) compared to placebo-treated patients (20%).
Increase in Liver Enzymes: Increases from baseline liver enzymes within the normal range were observed with plozasiran treatment in the FCS population. These increases occurred within the first 3 months of treatment and stabilized.
Increase in LDL-cholesterol: Increases in low-density lipoprotein cholesterol (LDL-C) and total apolipoprotein B (apoB) were observed in the FCS population treated with REDEMPLO compared to those treated with placebo [see Clinical Studies (14)]. Despite increases in the LDL-C, the average LDL-C value at Month 12 was less than 50 mg/dL in the 25 mg REDEMPLO group.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient data on REDEMPLO use in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Patients with FCS are at risk for pancreatitis during pregnancy because of defects in lipid metabolism and increased triglyceride levels (see Clinical Considerations).
In animal reproduction studies, no adverse drug-related developmental effects were observed in pregnant rats or rabbits with subcutaneous administration of plozasiran during organogenesis up to 23 and 140 times, respectively, the maximum recommended human dose (MRHD) (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20% respectively.
Disease-Associated Maternal and/or Embryo-Fetal Risk
Triglyceride levels increase during the third trimester of pregnancy. In patients with underlying defects in lipid metabolism, such as FCS, severe gestational hypertriglyceridemia may occur, increasing the risk of acute pancreatitis during pregnancy.
Animal Data
In an embryo-fetal development study, pregnant rats were administered plozasiran by subcutaneous injection at 0, 5, 15, or 60 mg/kg, or 60 mg/kg rat specific surrogate, once daily during the period of organogenesis (gestational days 6 to 17). There was no evidence of drug-related embryo-fetal toxicity or fetal malformations up to 60 mg/kg plozasiran [23 times the MRHD based on body surface area (BSA)]. At maternally toxic doses there were embryo-fetal toxicities including increases in post-implantation loss and mean number of late resorptions at 60 mg/kg (23 times the MRHD based on BSA), early deliveries, reduced fetal body weight, and fetal skeletal developmental variations at ≥15 mg/kg (6 times the MRHD based on BSA). No adverse embryo-fetal developmental effects were observed from a single subcutaneous administration of 50 mg/kg plozasiran (19 times the MRHD based on BSA) or the rat specific surrogate to pregnant rats on gestation day 10.
In an embryo-fetal development study in pregnant rabbits, plozasiran was administered by subcutaneous injection at 0, 30, 60, or 180 mg/kg/day once daily during the period of organogenesis (gestational days 7 to 19). No evidence (of embryo-fetal toxicity or developmental abnormalities) was observed up to 180 mg/kg (140 times the MRHD based on BSA).
In a rat pre- and post-natal development study, plozasiran was administered at 0, 8, 24, or 80 mg/kg by subcutaneous injection once a week from gestation day 6 through lactation day 17. Plozasiran increased the number of females with stillborn offspring and the increase in stillborn offspring per litter resulted in reductions in live birth index at 80 mg/kg (31 times the MRHD based on BSA). There were decreases in offspring body weight and offspring survival at ≥24 mg/kg (9 times the MRHD based on BSA). No adverse effects were noted on offspring development up to 80 mg/kg (31 times the MRHD based on BSA).
8.2 Lactation
Risk Summary
There is no information regarding the presence of plozasiran in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Oligonucleotide-based products typically have poor oral bioavailability. Therefore, it is considered that if plozasiran is present in breastmilk, it is unlikely to lead to clinically relevant levels in breastfed infants. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for REDEMPLO and any potential adverse effects on the breastfed infant from REDEMPLO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of REDEMPLO in pediatric patients with FCS have not been established.
8.5 Geriatric Use
Of the 75 patients with FCS randomized in Trial 1, 9 (12%) were 65 years of age or older, including 2 (3%) patients who were 75 years of age or older. No overall differences in safety or effectiveness of REDEMPLO have been observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
The recommended dosage of REDEMPLO in patients with mild or moderate renal impairment (eGFR ≥30 to <90 mL/min) is the same as those with normal renal function. The impact of severe renal impairment or end stage renal disease is not known [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The recommended dosage of REDEMPLO in patients with mild hepatic impairment [total bilirubin ≤1 times the upper limit of normal (ULN) and AST >1 times ULN, or total bilirubin >1.0 to 1.5 times ULN and any AST] is the same as those with normal hepatic function. The impact of moderate or severe hepatic impairment is not known [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
REDEMPLO contains plozasiran (present as plozasiran sodium), a small interfering RNA (siRNA) that degrades apolipoprotein C-III (apoC-III) mRNA by RNA interference. Plozasiran contains a covalently linked ligand containing three N-acetylgalactosamine (GalNAc) residues to facilitate delivery to hepatocytes. The 2´ positions of the ribose subunits in plozasiran are modified with either fluorine (2´F) or methoxy (2´O-Me) groups. Each strand of plozasiran also includes multiple phosphorothioates.
The molecular formula of plozasiran sodium is C493H611F11N164Na43O311P43S7 and its molecular weight is 16,563.98 Da. Plozasiran sodium is freely soluble in water. Plozasiran has the following structural formula:
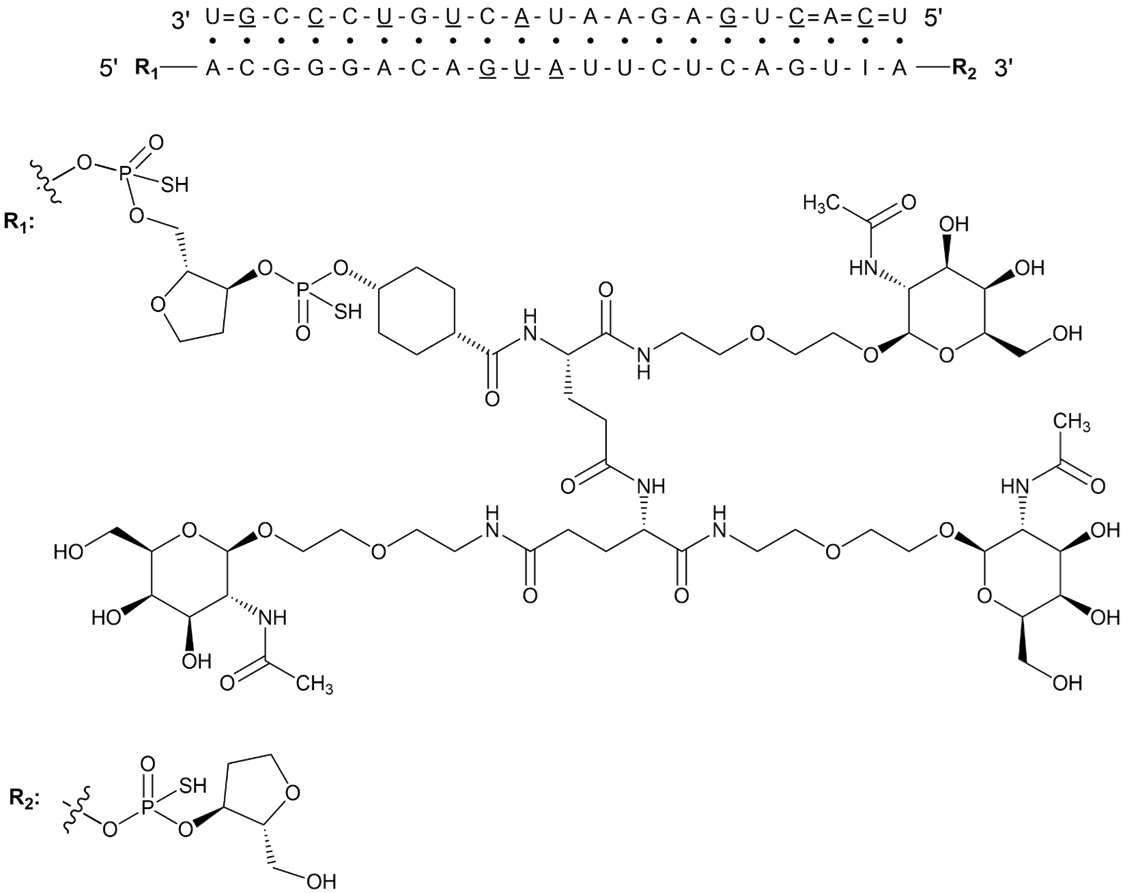
Abbreviations: A = 2’-O-methyladenosine; A = 2’-fluoro(2’-deoxy-2’-fluoro)adenosine; C = 2’-O-methylcytidine; C = 2’-fluorocytidine; G = 2’-O-methylguanosine; G = 2’-fluoroguanosine; I = 2’-O-methylinosine; U = 2’-O-methyluridine; U = 2’-fluorouridine; - (single line) = phosphodiester linkage; = (double line) = phosphorothioate linkage; · (middle dot) depicts base pairing between the two strands
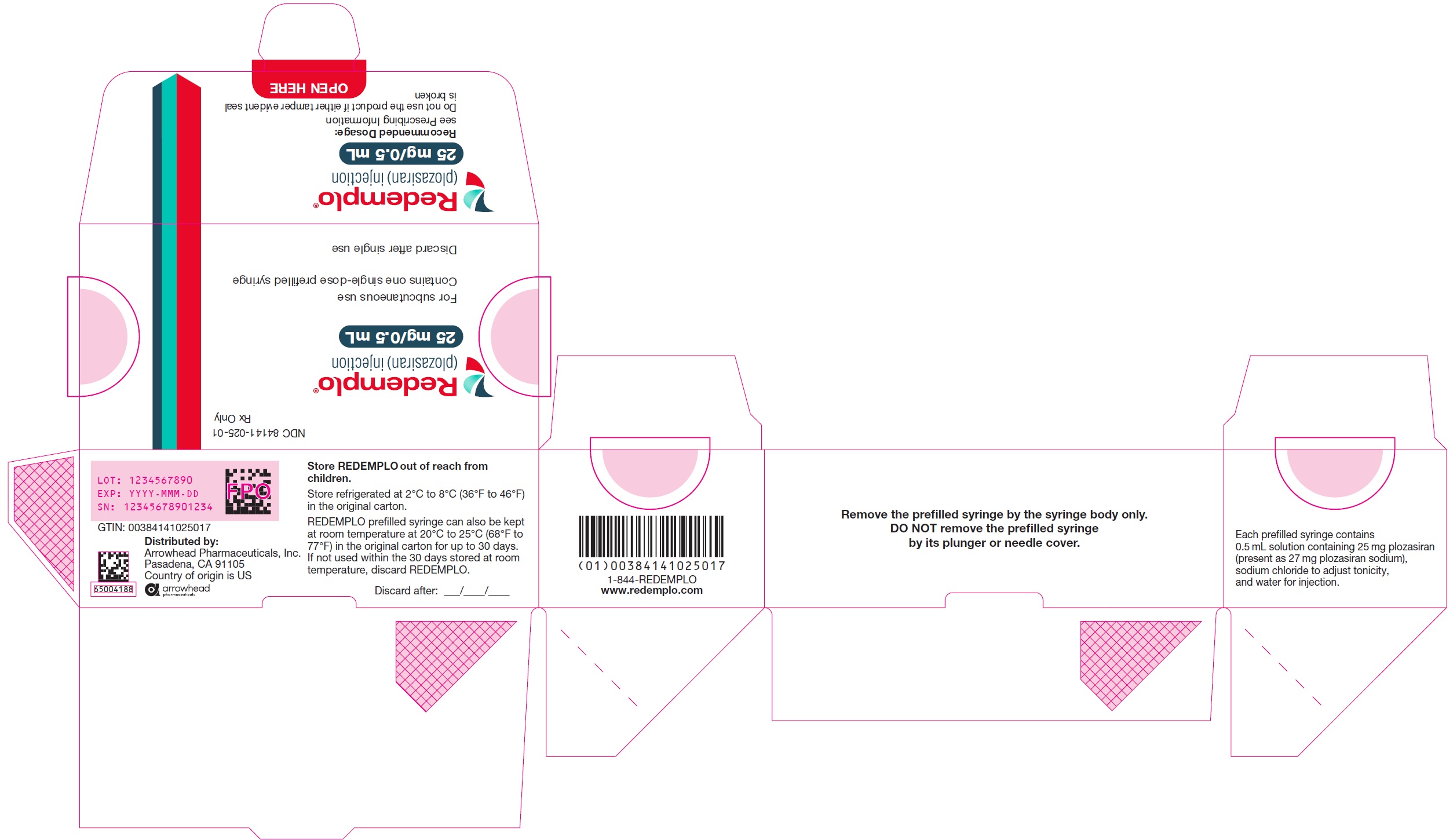
REDEMPLO is a sterile, preservative-free, clear, colorless to yellow solution for subcutaneous use in a prefilled syringe. Each syringe contains 0.5 mL of solution containing 25 mg plozasiran (present as 27 mg plozasiran sodium), sodium chloride to adjust tonicity, and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Plozasiran is a siRNA conjugated with GalNAc that degrades the apoC-III mRNA through the RNA interference mechanism resulting in reduced levels of hepatic and serum apoC-III protein. Reduction of apoC-III protein leads to increased clearance of serum triglycerides.
12.2 Pharmacodynamics
In Trial 1 [see Clinical Studies (14)], following the recommended dose of 25 mg administered every 3 months in patients with FCS, REDEMPLO reduced median fasting serum apoC-III protein. The placebo-corrected median percent change in fasting serum apoC-III protein from baseline was -90% at 1 month, -93% at 3 months, -82% at 6 months, -91% at 10 months, and -87% at 12 months.
Cardiac Electrophysiology
At a dose 4 times the recommended dose of 25 mg administered every 3 months, clinically significant QTc interval prolongation was not observed.
12.3 Pharmacokinetics
REDEMPLO exhibited linear and time-invariant pharmacokinetics following subcutaneous injections within the dose range of 10 mg to 100 mg. The following pharmacokinetic parameters were observed in healthy adults after receiving a 25 mg dose of REDEMPLO.
Absorption
Plozasiran peak plasma concentration (Cmax) is 68.5 ng/mL. The median time to reach Cmax (Tmax) is 6 hours.
Distribution
Plozasiran is 78% protein bound in vitro at the clinically relevant plasma concentrations. Following subcutaneous multiple administration of 25 mg plozasiran, the apparent volume of distribution is approximately 146 L. Plozasiran is distributed in plasma and extracellular body water before its uptake by hepatocytes to decrease apoC-III mRNA expression and reduce serum triglycerides.
Elimination
The terminal elimination half-life of plozasiran in plasma is approximately 3 to 4 hours. The mean apparent systemic clearance is 33.8 L/hour.
Metabolism
Plozasiran is primarily metabolized by nucleases to shorter oligonucleotides of varying lengths.
Excretion
Approximately 16 to 19% of REDEMPLO dose is excreted in urine.
Specific Populations
No clinically significant differences in plozasiran pharmacokinetics based on age, sex, race, mild and moderate renal impairment (eGFR ≥30 to <90 mL/min), or mild hepatic impairment (total bilirubin ≤1 times ULN and AST >1 times ULN, or total bilirubin >1.0 to 1.5 times ULN and any AST) were found in the population pharmacokinetic analysis. The impact of severe renal impairment, end-stage renal impairment, or moderate to severe hepatic impairment is not known.
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
CYP450 Enzymes
Plozasiran is not a substrate, inhibitor, or inducer of CYP450 enzymes at clinically relevant concentrations.
Transporter Systems
Plozasiran is not a substrate or an inhibitor of P-gp, BCRP, OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, or MATE2-K.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADAs) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADAs in the trial described below with the incidence of anti-drug antibodies in other studies, including those of plozasiran.
In Trial 1, none of the 50 FCS-patients treated with REDEMPLO over a period of 12 months developed treatment-induced or treatment-boosted ADAs. Because ADAs were not observed in the limited number of REDEMPLO-treated patients, the effect of ADAs on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of REDEMPLO products is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 26-week study in RasH2Tg mice, plozasiran was administered subcutaneously once every 8 weeks at dose levels of 30, 60, and 120 mg/kg. Plozasiran was not carcinogenic up to the highest tested dose of 120 mg/kg (23-times MRHD based on BSA).
Mutagenesis
Plozasiran was not mutagenic or clastogenic in a standard battery of genetic toxicity assays, including a bacterial mutation (Ames) assay, and in vitro and in vivo mouse micronucleus assays.
Impairment of Fertility
In a fertility and early embryonic-development study, male and female rats were administered subcutaneously with vehicle or plozasiran at the doses of 12.5, 25 or 50 mg/kg or rat specific surrogate at 25 mg/kg. Males were treated once weekly before and throughout cohabitation, while females received treatment either once every 3 days or once weekly before and through mating until gestation day 6. There were no adverse effects on mating and fertility in males or females up to 50 mg/kg corresponding to 19-times the MRHD, based on BSA.
-
14 CLINICAL STUDIES
The efficacy of REDEMPLO was demonstrated in a randomized, placebo-controlled, double-blind trial in adult patients with genetically confirmed or clinically diagnosed FCS maintained on a low-fat diet(≤20 grams fat per day) (Trial 1; NCT05089084). Patients were randomized to receive four total doses of REDEMPLO 25 mg (n=26) or matching placebo (n=25), injected subcutaneously once every 3 months over a 12-month treatment period
The diagnosis of FCS was based on adults with a screening fasting TG ≥880 mg/dL refractory to lipid-lowering therapy, with a history of elevated triglycerides (in excess of 1,000 mg/dL at least three times), and evidence of FCS by known genotypes, evidence of low lipoprotein lipase activity, or a clinical diagnosis. In this trial, for patients with clinically diagnosed FCS, the inclusion criteria specified at least one of the following: recurrent episodes of acute pancreatitis not caused by alcohol or cholelithiasis; recurrent hospitalizations for severe abdominal pain without other explainable cause; childhood pancreatitis; or family history of hypertriglyceridemia-induced pancreatitis.
Patient demographics were generally similar across the treatment groups [see Adverse Reactions (6.1)]. At enrollment, the percentage of patients with genetic confirmation of FCS was 46% in the REDEMPLO 25 mg group compared with 56% in the placebo group; diabetes was 15% in the REDEMPLO 25 mg group compared with 32% in the placebo group; and a history of documented acute pancreatitis in the prior 5 years was 54% in the REDEMPLO 25 mg group compared with 68% in the placebo group. Patients in the REDEMPLO 25 mg and placebo groups were treated with statins (43%), omega-3 fatty acids (29%), fibrates (69%), or no background TG lowering therapies (25%) at study entry. Mean (SD) and median fasting TG levels at baseline were 2,311 (1,258) mg/dL and 2,030 mg/dL, respectively (range of 747 to 5,596 mg/dL).
The primary efficacy endpoint was percent change in fasting triglycerides from baseline at Month 10 (average of 2 assessments, 2 to 7 days apart). The median difference between REDEMPLO 25 mg and the placebo group in percent change in fasting triglyceride levels from baseline to Month 10 was -58.7% (95% CI: -89.6, -27.9; p< 0.0001). For additional results see Table 2.
Table 2: Baseline and Percent Changes from Baseline in Lipid/Lipoprotein Parameters in Patients with FCS at Month 10 in Trial 1 Abbreviations: ApoB = apolipoprotein B; CI= confidence interval; BL = baseline; FCS=familial chylomicronemia syndrome; non-HDL-C = non-high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
a Reached statistical significance (p value ˂ 0.0001).
b Median; Hodges-Lehmann method was used to estimate the median difference (location shift) and its corresponding 95% confidence interval for percent changes. Missing data were imputed using washout imputation.
c Mean; Analysis of covariance (ANCOVA) model was used to estimate the mean difference and its corresponding 95% confidence interval for percent changes. Missing data were imputed using washout imputation.
REDEMPLO 25 mg
N=26Placebo (pooled)
N=25REDEMPLO 25 mg vs.
PlaceboParameter
(mg/dL)BL % change at
Month 10BL % change at
Month 10Treatment Difference
% change (95% CI) at
Month 10Triglyceridesbb 2008 -80 2053 -17 -59a
(-90, -28)Non-HDL-Cc 279 -39 268 4 -42
(-67, -18)LDL-Cc 24 112 28 20 92
(4, 180)Total ApoBc 72 27 79 12 15
(-16, 46)ApoB-48c 10 -61 11 45 -106
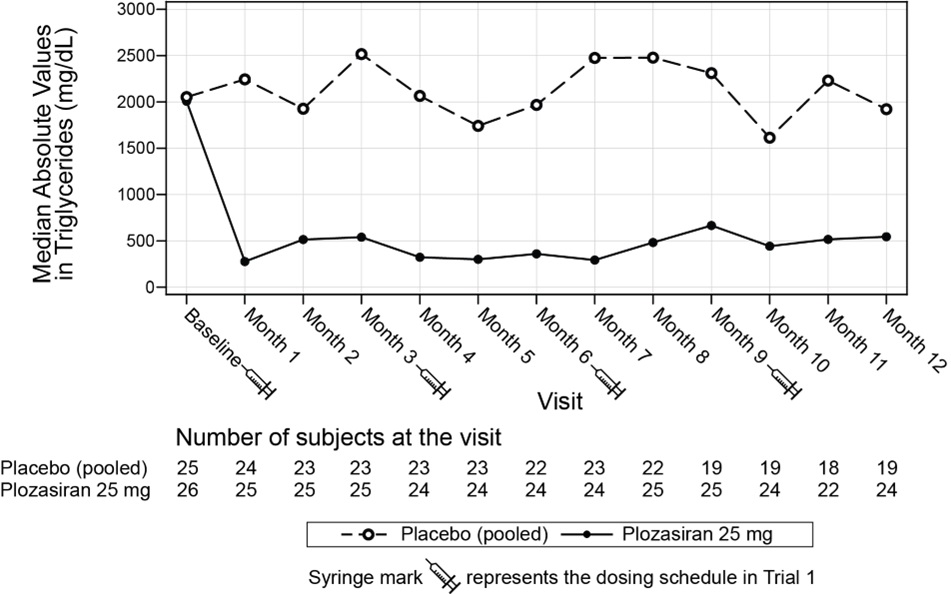
(-180, -33)Median percent change in TG from baseline (Figure 1) and median absolute TG values (Figure 2) over time demonstrated a consistent lowering effect during the 12-month treatment period.
Figure 1: Median Percent Change from Baseline in Fasting Triglycerides Over Time in Trial 1
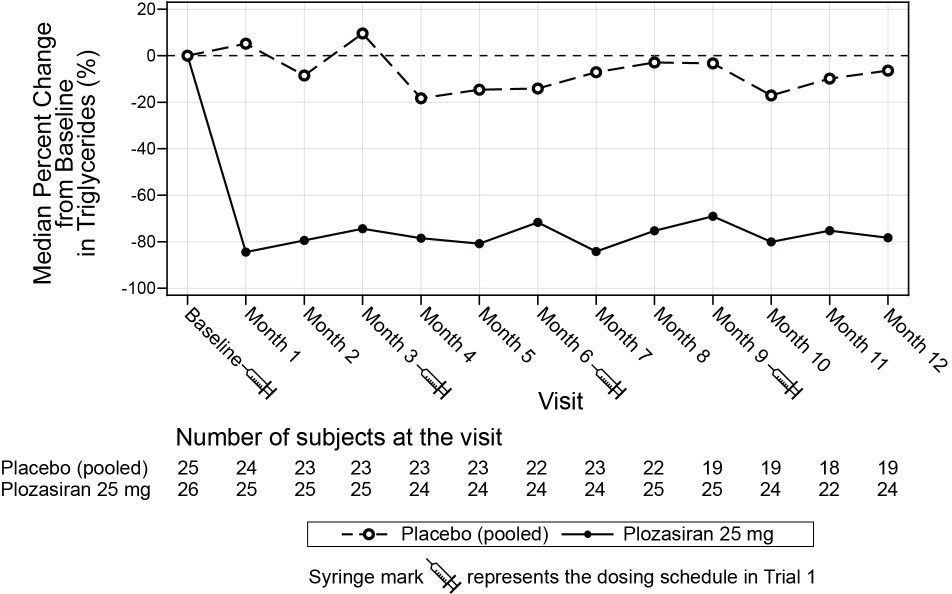
Figure 2: Median Absolute Fasting Triglyceride Levels (mg/dL) in Trial 1
Over the 12-month treatment period, the numerical incidence of acute pancreatitis in patients treated with REDEMPLO 25 mg was lower compared with placebo [2 (8%) patients in the REDEMPLO 25 mg group compared with 5 (20%) patients in the placebo group].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
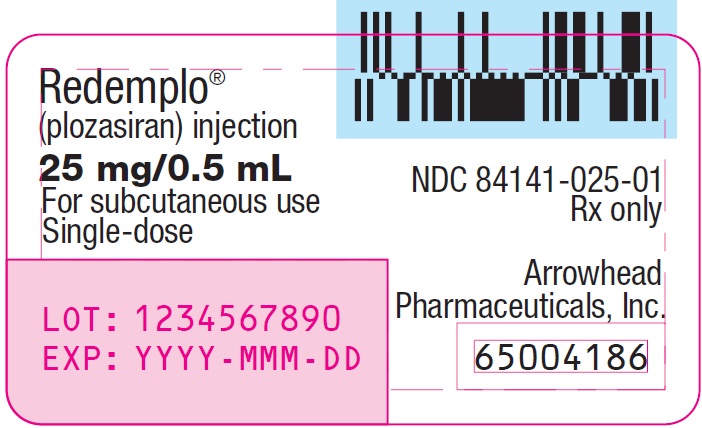
REDEMPLO injection is a clear and colorless to yellow solution supplied in a single-dose prefilled syringe. Each prefilled syringe of REDEMPLO is filled to deliver 0.5 mL of solution containing 25 mg of plozasiran.
REDEMPLO is available in cartons containing one 25 mg single-dose prefilled syringe each (NDC: 84141-025-01).
Storage
Store REDEMPLO refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton, until ready for use.
REDEMPLO prefilled syringe can also be kept at room temperature at 20°C to 25°C (68°F to 77°F) in the original carton for up to 30 days. If not used within the 30 days stored at room temperature, discard REDEMPLO.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Adherence to Diet
Advise patients with FCS that use of lipid-regulating agents does not reduce the importance of adhering to a low-fat diet (less than or equal to 20 grams fat per day) [see Dosage and Administration (2.2)].
Missed Dose
Instruct patients to take REDEMPLO as prescribed. If a dose is missed, instruct patients to take as soon as they remember. Resume dosing every 3 months from the date of the most recently administered dose [see Dosage and Administration (2.2)].
Distributed by:
Arrowhead Pharmaceuticals, Inc.
Pasadena, CA 91105
© 2025, Arrowhead Pharmaceuticals, Inc.
All rights reserved.
REDEMPLO is a registered trademark of Arrowhead Pharmaceuticals, Inc.
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Approved: 11/2025 PATIENT INFORMATION
REDEMPLO® [ree-DEM-plo]
(plozasiran)
injection, for subcutaneous useRead this Patient Information carefully before you start taking REDEMPLO. If you have any questions about REDEMPLO, ask your healthcare provider.
What is REDEMPLO?
- REDEMPLO is an injectable prescription medicine used together with a low-fat diet to reduce triglycerides (fat in the blood) in adults with a condition that keeps the body from breaking down fats called familial chylomicronemia syndrome (FCS).
- It is not known if REDEMPLO is safe and effective in children.
Before using REDEMPLO, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if REDEMPLO can harm your unborn baby. Tell your healthcare provider if you become pregnant while using REDEMPLO.
- are breastfeeding or plan to breastfeed. It is not known if REDEMPLO passes into your breast milk and if it can harm your baby. Talk with your healthcare provider about the best way to feed your baby while using REDEMPLO.
Tell your healthcare provider about all of the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use REDEMPLO?
- Read the Instructions for Use that comes with your REDEMPLO pre-filled syringe.
- Your healthcare provider will show you and/or your caregiver(s) how to inject REDEMPLO the first time.
- REDEMPLO is injected under the skin (subcutaneous use) in the front of your upper legs (thighs) or in your stomach area (abdomen). Only your healthcare provider or caregiver(s) may give you an injection in the outer area of your upper arm.
- REDEMPLO should be injected 1 time every 3 months.
- If you miss a dose, take the missing dose as soon as possible. Then inject REDEMPLO 3 months from the date of your last dose to get back on the every-3-month dosing schedule.
- Stay on a low-fat diet (less than or equal to 20 grams of fat each day) while using REDEMPLO.
What are the possible side effects of REDEMPLO?
The most common side effects of REDEMPLO include:
- high blood sugar (hyperglycemia)
- headache
- nausea
- injection site reaction (pain, redness, or swelling)
These are not all of the possible side effects of REDEMPLO. Tell your healthcare provider if you have any of the above side effects. Call your health care provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or by calling 1-844-REDEMPLO (1-844-733-3675) or via https://arrowheadpharma.com/safetyreporting.
How should I store REDEMPLO?
- Store REDEMPLO prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original carton, until you are ready to use.
- REDEMPLO can also be stored at room temperature between 68°F to 77°F (20°C to 25°C) in the original carton for up to 30 days.
- Throw away the REDEMPLO prefilled syringe if kept at room temperature longer than 30 days.
General information about the safe and effective use of REDEMPLO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use REDEMPLO for a condition for which it was not prescribed. Do not give REDEMPLO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about REDEMPLO that is written for health professions.
What are the ingredients in REDEMPLO?
Active ingredients: plozasiran sodium
Inactive ingredients: sodium chloride, water for injection
Distributed by:
Arrowhead Pharmaceuticals, Inc.
Pasadena, CA 91105
©2025, Arrowhead Pharmaceuticals, Inc.
All rights reserved.
-
INSTRUCTIONS FOR USE
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Approved: 11/2025 INSTRUCTIONS FOR USE
REDEMPLO® [ree-DEM-plo]
(plozasiran)
injection, for subcutaneous use
single-dose prefilled syringe
25 mg/0.5 mLThis Instructions for Use contains information on how to inject REDEMPLO (plozasiran).
Read this Instructions for Use before you start using your REDEMPLO prefilled syringe and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. Your healthcare provider should show you or your caregiver how to use your REDEMPLO prefilled syringe the right way. Call your healthcare provider or pharmacist if you have any questions.Guide to Parts
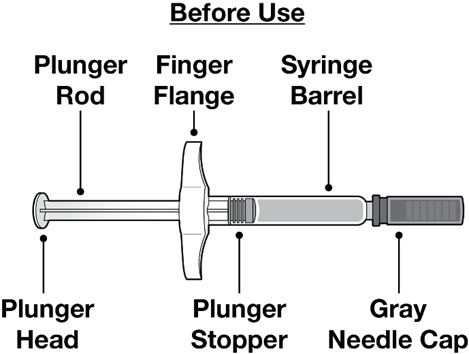
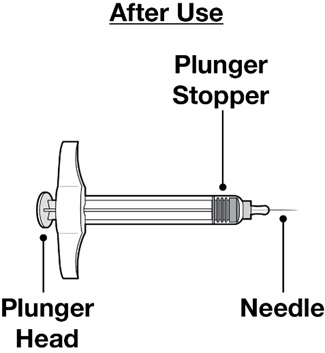
Each carton contains a single-dose REDEMPLO (plozasiran) Injection prefilled syringe for single use under the skin (subcutaneous) injection.
Important Information You Need to Know Before Injecting REDEMPLO
- REDEMPLO is for subcutaneous injection only
- Each carton contains a 1-time (single-dose) REDEMPLO (plozasiran) prefilled syringe.
- Do not use your REDEMPLO single-dose prefilled syringe if the carton appears damaged or the tamper evident seal on the carton is broken.
- Do not remove the gray needle cap until you are ready to inject (Step 6).
- Do not use if the single-dose prefilled syringe appears damaged.
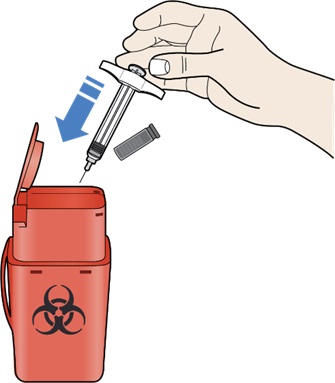
- Throw away (dispose of) your REDEMPLO prefilled syringe in a sharps disposal container right away after use (Step 10: Disposing of REDEMPLO).
Storing REDEMPLO
- Keep the REDEMPLO prefilled syringe in the original carton until ready to use.
- Store the REDEMPLO prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original carton.
- REDEMPLO prefilled syringe can also be stored at room temperature between 68°F to 77°F (20°C to 25°C) in the original carton for up to 30 days.
- Do not let REDEMPLO reach temperatures above 77°F (25°C).
- Throw away the REDEMPLO prefilled syringe if kept at room temperature and not used within 30 days.
If the product is not stored in any of the above conditions, throw away the prefilled syringe in a sharps disposal container and use a new prefilled syringe.
Keep REDEMPLO prefilled syringe and all medicine out of reach of children.
Preparing to Inject REDEMPLO
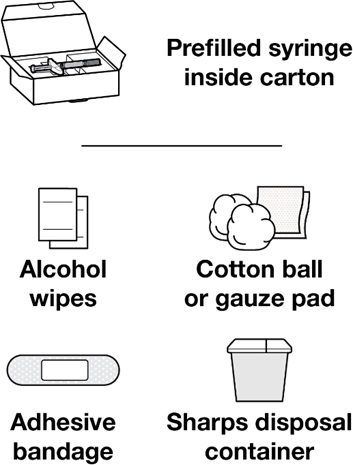
Step 1: Gather all materials needed for your REDEMPLO injection
Figure A
On a clean, well-lit, flat work surface, place:
Provided in the carton (see Figure A):- 1 REDEMPLO prefilled syringe
Other materials not provided (see Figure A):
- Alcohol wipes
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container
Step 2: Prepare to use REDEMPLO prefilled syringe
Figure B
Figure C
Figure D
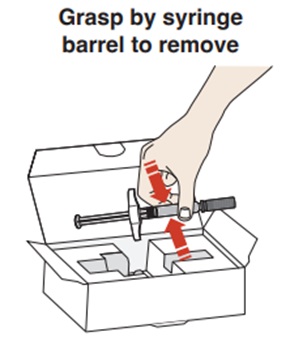
- If refrigerated, remove the carton from the refrigerator.
- Open the carton lid and remove the syringe by the syringe barrel and place on a flat surface (see Figure B).
- Do not use the prefilled syringe if tamper evident seal on the carton is broken.
- Do not pick up or pull the prefilled syringe by the plunger rod or gray needle cap.
-
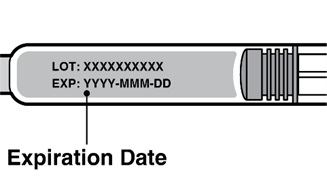
Check the expiration date on the REDEMPLO prefilled syringe. (see Figure C).
- Do not use if the expiration date has passed.
- Wait 30 minutes for the prefilled syringe to reach room temperature before injecting (see Figure D).
- Do not try to warm the prefilled syringe by using a heat source such as hot water or a microwave.
- Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
Step 3: Check the medicine and syringe
Figure E
Figure F
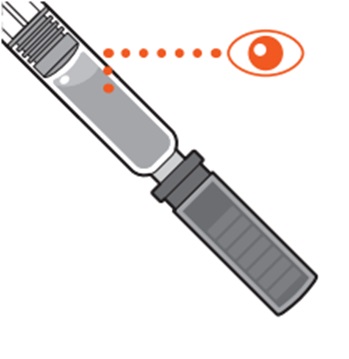
- Check the the REDEMPLO medicine in the prefilled syringe (see Figure E).
- The medicine should be clear and colorless to yellow.
- Do not use the prefilled syringe if the medicine is cloudy, discolored or contains particles.
- It is normal to see air bubbles in the medicine.
- The medicine should be clear and colorless to yellow.
- Inspect the prefilled syringe (see Figure F).
- Do not use the prefilled syringe if any part appears cracked or broken.
- Do not use the prefilled syringe if the gray needle cap is missing or not securely attached.
- Do not use the prefilled syringe if it has been dropped onto a hard surface because the syringe may be damaged.
- In any of the above cases, call 1-844-REDEMPLO or visit www.redemplo.com.
Injecting REDEMPLO
Step 4: Choose your injection site
Figure G
-
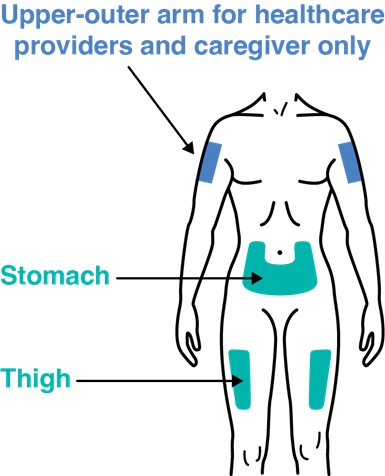
Choose an injection site on the (see Figure G):
- Upper leg (thigh) or
- Stomach (abdomen) except for a 2-inch area right around the belly button (navel).
Only your healthcare providers or caregivers can use the outer area of the upper arm (see Figure G).
-
Do not inject:
- within 2 inches of the belly button (navel).
- into skin that is damaged (tender, bruised, red, hard, or cut).
- into areas with scars, stretch marks.
Step 5: Wash hands and clean the injection site
Figure H
Figure I
Figure J
- Hold the prefilled syringe by the syringe barrel, with the needle facing away from you.
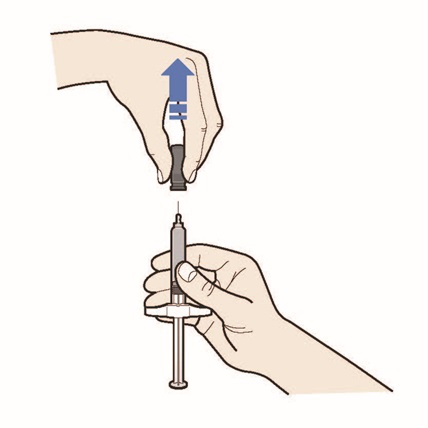
- Pull the gray needle cap straight up and away from your body (see Figure J).
- Do not twist or bend the gray needle cap.
- Avoid pushing the plunger head before you are ready to inject.
- Do not let the needle touch any surface.
- Do not put the needle cap back onto your prefilled syringe.
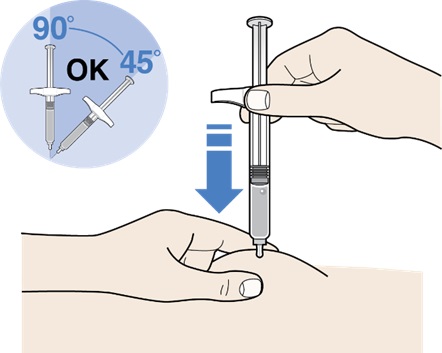
Step 7: Pinch the skin and Insert the needle
Figure K
- Hold your prefilled syringe by the finger flange in one hand.
- Gently pinch and hold a fold of skin at the injection site with your other hand.
- Insert needle at a 45°(degree) to 90° angle (see Figure K).
-
Keep the skin pinched while you are inserting the needle and during injection.
- Do not place your finger on the plunger head before the injection.
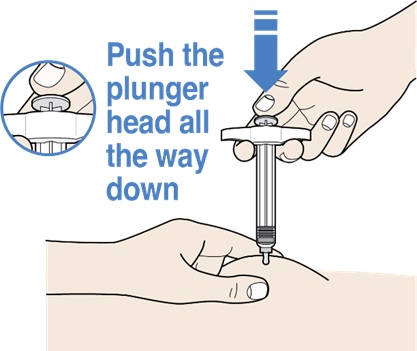
Step 8: Hold skin pinch and Push down the plunger head
Figure L
- While pinching the skin, push the plunger head all the way down using slow and constant pressure (see Figure L).
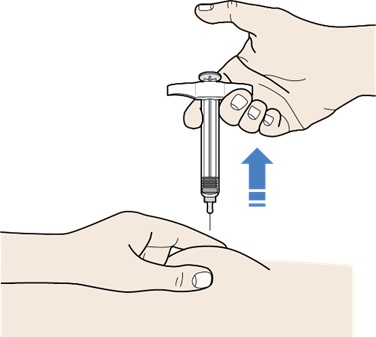
Step 9: Complete the Injection
Figure M
- After the plunger head is pushed all the way down, take the needle out of the skin, by gently lifting the syringe up and off the skin until the needle is completely removed (see Figure M).
- Do not pull the plunger head up. Lift the whole syringe straight up.
- Do not rub the injection site.
- There may be a small amount of blood or liquid where you injected. This is normal.
- If needed, press a cotton ball or gauze pad on the area and apply an adhesive bandage.
- Place the used cap and syringe in a sharps disposal container right away (see section – “Disposing of REDEMPLO”).
- Do not put the needle cap back onto the syringe.
Figure N
Do not use any medicine that is left in the used syringe.
- Put your used syringes and needle cap in an FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) loose needles and syringes in your household trash (see Figure N).
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
- For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
For more information, call 1-844-REDEMPLO or visit www.redemplo.com.
Distributed by:
Arrowhead Pharmaceuticals, Inc.
Pasadena, CA 91105
Phone Number: 1-844-REDEMPLO
- PRINCIPAL DISPLAY PANEL - NDC: 84141-025-01 - Carton Label - 25mg/0.5mL
- PRINCIPAL DISPLAY PANEL - NDC: 84141-025-01 - Container Label - 25mg/0.5mL
-
INGREDIENTS AND APPEARANCE
REDEMPLO
plozasiran injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 84141-025 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PLOZASIRAN (UNII: XG9ARL6P25) (PLOZASIRAN - UNII:XG9ARL6P25) PLOZASIRAN 25 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 84141-025-01 1 in 1 CARTON 11/19/2025 1 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219947 11/19/2025 Labeler - Arrowhead Pharmaceuticals, Inc. (607735859)
Trademark Results [REDEMPLO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REDEMPLO 98355091 not registered Live/Pending |
ARROWHEAD PHARMACEUTICALS, INC. 2024-01-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.