PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT- original and omicron ba.4/ba.5 injection, suspension
Pfizer Manufacturing Belgium NV
----------
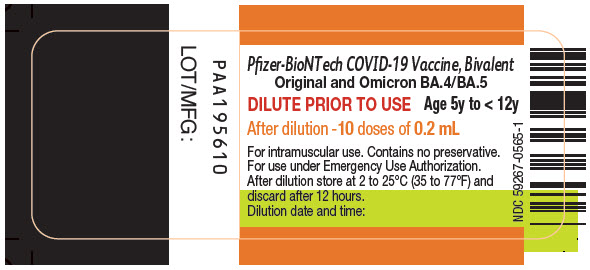
Pfizer-BioNTech COVID-19 Vaccine, Bivalent
Original and Omicron BA.4/BA.5
PRINCIPAL DISPLAY PANEL
Image not
available
PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT
original and omicron ba.4/ba.5 injection, suspension |
| Product Information |
| Product Type | VACCINE | Item Code (Source) | NDC: 59267-0609 |
| Route of Administration | INTRAMUSCULAR |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| TOZINAMERAN (UNII: 5085ZFP6SJ) (TOZINAMERAN - UNII:5085ZFP6SJ) | TOZINAMERAN | 0.02 mg in 2.6 mL |
| BNT162B2 OMICRON (BA.4/BA.5) (UNII: JSV288Q5CV) (BNT162B2 OMICRON (BA.4/BA.5) - UNII:JSV288Q5CV) | BNT162B2 OMICRON (BA.4/BA.5) | 0.02 mg in 2.6 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| 2-(MPEG 2000)-N,N-DITETRADECYLACETAMIDE (UNII: PJH39UMU6H) | 0.07 mg in 2.6 mL |
| ((4-HYDROXYBUTYL)AZANEDIYL)BIS(HEXANE-6,1-DIYL)BIS(2-HEXYLDECANOATE) (UNII: AVX8DX713V) | 0.57 mg in 2.6 mL |
| TROMETHAMINE (UNII: 023C2WHX2V) | 0.08 mg in 2.6 mL |
| TROMETHAMINE HYDROCHLORIDE (UNII: 383V75M34E) | 0.53 mg in 2.6 mL |
| SUCROSE (UNII: C151H8M554) | 41.2 mg in 2.6 mL |
| 1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOCHOLINE (UNII: 043IPI2M0K) | 0.12 mg in 2.6 mL |
| CHOLESTEROL (UNII: 97C5T2UQ7J) | 0.25 mg in 2.6 mL |
| WATER (UNII: 059QF0KO0R) | |
| SODIUM CHLORIDE (UNII: 451W47IQ8X) | 19.8 mg in 2.6 mL |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 59267-0609-2 | 10 in 1 CARTON | | |
| 1 | NDC: 59267-0609-1 | 2.6 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| EMERGENCY USE AUTHORIZATION | | 08/15/2022 | 08/15/2022 |
|
| Labeler - Pfizer Manufacturing Belgium NV
(370156507)
|
| Registrant - Pfizer Inc (113480771) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| BioNTech Innovative Manufacturing Services GmbH | | 537365801 | ANALYSIS(59267-0609) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| BioNTech Manufacturing GmbH | | 314382536 | ANALYSIS(59267-0609) , API MANUFACTURE(59267-0609) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| BioNTech Manufacturing Marburg GmbH | | 313270335 | ANALYSIS(59267-0609) , API MANUFACTURE(59267-0609) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC | | 174350868 | ANALYSIS(59267-0609) , API MANUFACTURE(59267-0609) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| Pfizer Inc | | 004954111 | ANALYSIS(59267-0609) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| Pfizer Ireland Pharmaceuticals | | 985586408 | ANALYSIS(59267-0609) |