FLUDARABINE PHOSPHATE injection
Fludarabine Phosphate by
Drug Labeling and Warnings
Fludarabine Phosphate by is a Prescription medication manufactured, distributed, or labeled by Areva Pharmaceuticals, NerPharMa SRL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Fludarabine Phosphate safely and effectively. See full prescribing information for Fludarabine Phosphate.
Fludarabine Phosphate Injection, for intravenous use only
Initial U.S. Approval 1991WARNING: CNS TOXICITY, HEMOLYTIC ANEMIA, AND PULMONARY TOXICITY
See full prescribing information for complete boxed warning.
- Severe central nervous system toxicity occurred in 36% of patients treated with doses approximately four times greater (96 mg/m 2/day for 5 to 7 days) than the recommended dose. This toxicity was seen in ≤0.2% of patients treated at the recommended dose levels (25 m g/m 2). (5.1)
- Instances of life-threatening and sometimes fatal autoimmune hemolytic anemia have been reported after one or more cycle s of treatment. (5.3)
- In a clinical investigation of the combination of fludarabine phosphate with pentostatin (deoxycoformycin) for the treatment of refractory chronic lymphocytic leukemia (CLL), there was an unacceptably high incidence of fatal pulmonary toxicity. (5.5)
INDICATIONS AND USAGE
Fludarabine Phosphate Injection is a nucleotide metabolic inhibitor indicated for: (1)
The treatment of adult patients with B-cell chronic lymphocytic leukemia (CLL) who have not responded to or whose disease has progressed during treatment with at least one standard alkylating-agent containing regimen. Benefit in treatment-naïve or non-refractory CLL patients is not established. (1.1) (1)
(1)
DOSAGE AND ADMINISTRATION
- The recommended adult dose is 25 mg/m 2 administered intravenously over a period of approximately 30 minutes daily for five consecutive days. Each 5 day course of treatment should commence every 28 days. (2.1)
- Reduce dose in patients with creatinine clearance 30 to 70 mL/min/l.73 m 2. Do not use in patients with severe renal impairment (2.2).
DOSAGE FORMS AND STRENGTHS
Fludarabine Phosphate Injection is supplied as a 50 mg per 2 mL (25 mg per mL) sterile solution. (3) (3)
CONTRAINDICATIONS
- None
WARNINGS AND PRECAUTIONS
- Severe bone marrow suppression, notably anemia, thrombocytopenia and neutropenia. Monitor blood counts before and during treatment. (5.2)
- Transfusion-associated graft-versus-host disease. Use only irradiated blood products for transfusions. (5.4)
- Infections. Monitor for infection. (5.2)
- Renal Insufficiency. Reduce dose for moderate renal impairment and monitor closely. Do not administer to patients with severe renal impairment. (5.9)
- Tumor lysis syndrome (TLS). Take precautions for patients at high risk for TLS. (5.8)
- Can cause fetal harm when administered to a pregnant woman. Women should be advised to avoid becoming pregnant. (5.6)
ADVERSE REACTIONS
Most common adverse reactions (incidence > 30%) include myelosuppression (neutropenia, thrombocytopenia and anemia), fever, infection, nausea and vomiting, fatigue, anorexia, cough and weakness (6).
To report SUSPECTED ADVERSE REACTIONS, contact Areva at 1-855-853-4760 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Fludarabine phosphate injection in combination with pentostatin is not recommended due to the risk of severe pulmonary toxicity (5.5 and 7.1).
USE IN SPECIFIC POPULATIONS
Revised: 1/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CNS TOXICITY, HEMOLYTIC ANEMIA, AND PULMONARY TOXICITY
1 INDICATIONS AND USAGE
1.1 Indication
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Renal Impairment
2.3 Use of Infusion Solutions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Dose Dependent Neurologic Toxicities
5.2 Bone Marrow Suppression
5.3 Autoimmune Reactions
5.4 Transfusion Associated Graft-Versus-Host Disease
5.5 Pulmonary Toxicity
5.6 Pregnancy
5.7 Male Fertility and Reproductive Outcomes
5.8 Tumor Lysis
5.9 Renal Impairment
5.10 Vaccination
6 ADVERSE REACTIONS
6.1 Hematopoietic Systems
6.2 Infections
6.3 Metabolic
6.4 Nervous System
6.5 Pulmonary System
6.6 Gastrointestinal System
6.7 Cardiovascular
6.8 Genitourinary System
6.9 Skin
6.10 Neoplasms
6.11 Hepatobiliary Disorders
6.12 Adverse Reactions from Clinical Trials
7 DRUG INTREACTIONS
7.1 Pentostatin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.6 Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adults
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
16.3 Handling and Disposal
17.1 Monitoring
17.2 Laboratory Tests
17.3 Pregnancy
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CNS TOXICITY, HEMOLYTIC ANEMIA, AND PULMONARY TOXICITY
WARNING: SEVERE BONE MARROW SUPPRESSION, CNS TOXICITY, HEMOLYTIC ANEMIA, AND PULMONARY TOXICITY
Fludarabine Phosphate Injection should be administered under the supervision of a qualified physician experienced in the use of antineoplastic therapy. Fludarabine phosphate injection can severely suppress bone marrow function. When used at high doses in dose-ranging studies in patients with acute leukemia, fludarabine phosphate was associated with severe neurologic effects, including blindness, coma, and death. This severe central nervous system toxicity occurred in 36% of patients treated with doses approximately four times greater (96 mg/m 2/day for 5 to 7 days) than the recommended dose. Similar severe central nervous system toxicity, including coma, seizures, agitation and confusion, has been reported in patients treated at doses in the range of the dose recommended for chronic lymphocytic leukemia [see Warnings and Precautions (5.2)].
Instances of life-threatening and sometimes fatal autoimmune phenomena such as hemolytic anemia, autoimmune thrombocytopenia/thrombocytopenic purpura (ITP), Evans syndrome, and acquired hemophilia have been reported to occur after one or more cycles of treatment with Fludarabine Phosphate Injection. Patients undergoing treatment with Fludarabine Phosphate Injection should be evaluated and closely monitored for hemolysis [see Warnings and Precautions (5.3)].
In a clinical investigation using fludarabine phosphate in combination with pentostatin (deoxycoformycin) for the treatment of refractory chronic lymphocytic leukemia (CLL), there was an unacceptably high incidence of fatal pulmonary toxicity. Therefore, the use of Fludarabine Phosphate Injection in combination with pentostatin is not recommended [see Warnings and Precautions (5.5)].
-
1 INDICATIONS AND USAGE
1.1 Indication
Fludarabine Phosphate Injection is indicated for the treatment of adult patients with B-cell chronic lymphocytic leukemia (CLL) who have not responded to or whose disease has progressed during treatment with at least one standard alkylating-agent containing regimen. The safety and effectiveness of Fludarabine Phosphate Injection in previously untreated or non-refractory patients with CLL have not been established.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended adult dose of fludarabine phosphate injection is 25 mg/m 2 administered intravenously over a period of approximately 30 minutes daily for five consecutive days. Each 5-day course of treatment should commence every 28 days. Dosage may be decreased or delayed based on evidence of hematologic or nonhematologic toxicity. Physicians should consider delaying or discontinuing the drug if neurotoxicity occurs.
A number of clinical settings may predispose to increased toxicity from Fludarabine Phosphate Injection. These include advanced age, renal impairment, and bone marrow impairment. Such patients should be monitored closely for excessive toxicity and the dose modified accordingly.
The optimal duration of treatment has not been clearly established. It is recommended that three additional cycles of Fludarabine Phosphate Injection be administered following the achievement of a maximal response and then the drug should be discontinued.
2.2 Renal Impairment
Adjustments to the starting dose are recommended to provide appropriate drug exposure in patients with creatinine clearance 30 to 79 mL/min, as estimated by the Cockroft-Gault equations. These adjustments are based on a pharmacokinetic study in patients with renal impairment. Fludarabine Phosphate Injection should not be administered to patients with creatinine clearance less than 30 mL/min.
Starting Dose Adjustment for Renal Impairment
Creatinine Clearance
Starting Dose
≥ 80 mL/min
25 mg/m 2 (full dose)
50 to 79 mL/min
20 mg/m 2
30 to 49 mL/min
15 mg/m 2
< 30 mL/min
Do not administer
Renally impaired patients should be monitored closely for excessive toxicity and the dose modified accordingly.
2.3 Use of Infusion Solutions
Fludarabine Phosphate Injection contains no antimicrobial preservative and should be used within 8 hours of opening. Care must be taken to assure sterility of infusion solutions. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Fludarabine Phosphate Injection should not be mixed with other drugs.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
( see BOXED WARNINGS)
5.1 Dose Dependent Neurologic Toxicities
There are clear dose dependent toxic effects seen with fludarabine phosphate. Dose levels approximately 4 times greater (96 mg/m 2/day for 5 to 7 days) than that recommended for CLL (25 mg/m 2/day for 5 days) were associated with a syndrome characterized by delayed blindness, coma and death. Symptoms appeared from 21 to 60 days following the last dose. Thirteen of 36 patients (36%) who received fludarabine phosphate at high doses (96 mg/m 2/day for 5 to 7 days) developed this severe neurotoxicity. Similar severe central nervous system toxicity, including coma, seizures, agitation and confusion, has been reported in patients treated at doses in the range of the dose recommended for chronic lymphocytic leukemia.
In post-marketing experience neurotoxicity has been reported to occur either earlier or later than in clinical trials (range 7 to 225 days).
The effect of chronic administration of fludarabine phosphate on the central nervous system is unknown; however, patients have received the recommended dose for up to 15 courses of therapy.
Fludarabine phosphate may reduce the ability to drive or use mechanical equipment, since fatigue, weakness, visual disturbances, confusion, agitation and seizures have been observed.
5.2 Bone Marrow Suppression
Severe bone marrow suppression, notably anemia, thrombocytopenia and neutropenia, has been reported in patients treated with fludarabine phosphate. In a Phase I study in adult solid tumor patients, the median time to nadir counts was 13 days (range, 3 to 25 days) for granulocytes and 16 days (range, 2 to 32 days) for platelets. Most patients had hematologic impairment at baseline either as a result of disease or as a result of prior myelosuppressive therapy. Cumulative myelosuppression may be seen. While chemotherapy-induced myelosuppression is often reversible, administration of Fludarabine Phosphate Injection requires careful hematologic monitoring.
Several instances of trilineage bone marrow hypoplasia or aplasia resulting in pancytopenia, sometimes resulting in death, have been reported in adult patients. The duration of clinically significant cytopenia in the reported cases has ranged from approximately 2 months to approximately 1 year. These episodes have occurred both in previously treated or untreated patients.
5.3 Autoimmune Reactions
Instances of life-threatening and sometimes fatal autoimmune phenomena such as hemolytic anemia, autoimmune thrombocytopenia/thrombocytopenic purpura (ITP), Evans syndrome, and acquired hemophilia have been reported to occur after one or more cycles of treatment with fludarabine phosphate in patients with or without a previous history of autoimmune hemolytic anemia or a positive Coombs' test and who may or may not be in remission from their disease. Steroids may or may not be effective in controlling these hemolytic episodes. The majority of patients rechallenged with fludarabine phosphate developed a recurrence in the hemolytic process. The mechanism(s) which predispose patients to the development of this complication has not been identified. Patients undergoing treatment with Fludarabine Phosphate Injection should be evaluated and closely monitored for hemolysis. Discontinuation of therapy with Fludarabine Phosphate Injection is recommended in case of hemolysis.
5.4 Transfusion Associated Graft-Versus-Host Disease
Transfusion-associated graft-versus-host disease has been observed after transfusion of non-irradiated blood in fludarabine phosphate treated patients. Fatal outcome as a consequence of this disease has been reported. Therefore, to minimize the risk of transfusion-associated graft-versus-host disease, patients who require blood transfusion and who are undergoing, or who have received, treatment with Fludarabine Phosphate Injection should receive irradiated blood only.
5.5 Pulmonary Toxicity
In a clinical investigation using fludarabine phosphate in combination with pentostatin (deoxycoformycin) for the treatment of refractory chronic lymphocytic leukemia (CLL) in adults, there was an unacceptably high incidence of fatal pulmonary toxicity. Therefore, the use of Fludarabine Phosphate Injection in combination with pentostatin is not recommended.
5.6 Pregnancy
Based on its mechanism of action, fludarabine phosphate can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of Fludarabine Phosphate Injection in pregnant women. Fludarabine phosphate was embryolethal and teratogenic in rats and rabbits. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant [see Use in Specific Populations (8.1)].
5.7 Male Fertility and Reproductive Outcomes
Males with female sexual partners of childbearing potential should use contraception during and after cessation of fludarabine phosphate therapy. Fludarabine phosphate may damage testicular tissue and spermatozoa. Possible sperm DNA damage raises concerns about loss of fertility and genetic abnormalities in fetuses. The duration of this effect is uncertain [see Nonclinical Toxicology (13.1)].
5.8 Tumor Lysis
Tumor lysis syndrome has been associated with fludarabine phosphate treatment. This syndrome has been reported in CLL patients with large tumor burdens. Since fludarabine phosphate can induce a response as early as the first week of treatment, precautions should be taken in those patients at risk of developing this complication.
5.9 Renal Impairment
Fludarabine Phosphate Injection must be administered cautiously in patients with renal impairment. The total body clearance of 2-fluoro-ara-A has been shown to be directly correlated with creatinine clearance. Patients with creatinine clearance 30 to 79 mL/min should have their fludarabine phosphate dose reduced and be monitored closely for excessive toxicity. Fludarabine phosphate should not be administered to patients with creatinine clearance less than 30 mL/min [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
In patients aged 65 years or older, creatinine clearance should be measured before start of treatment.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Very common adverse reactions include myelosuppression (neutropenia, thrombocytopenia and anemia), fever and chills, fatigue, weakness, infection, pneumonia, cough, nausea, vomiting and diarrhea. Other commonly reported events include malaise, mucositis, and anorexia. Serious opportunistic infections have occurred in CLL patients treated with fludarabine phosphate. The most frequently reported adverse reactions and those reactions which are more clearly related to the drug are arranged below according to body system.
6.1 Hematopoietic Systems
Hematologic events (neutropenia, thrombocytopenia, and/or anemia) were reported in the majority of CLL patients treated with fludarabine phosphate. During fludarabine phosphate treatment of 133 patients with CLL, the absolute neutrophil count decreased to less than 500/mm 3 in 59% of patients, hemoglobin decreased from pretreatment values by at least 2 grams percent in 60%, and platelet count decreased from pretreatment values by at least 50% in 55%. Myelosuppression may be severe, cumulative, and may affect multiple cell lines. Bone marrow fibrosis occurred in one CLL patient treated with fludarabine phosphate.
Several instances of trilineage bone marrow hypoplasia or aplasia resulting in pancytopenia, sometimes resulting in death, have been reported in post-marketing surveillance. The duration of clinically significant cytopenia in the reported cases has ranged from approximately 2 months to approximately 1 year. These episodes have occurred both in previously treated or untreated patients.
Life-threatening and sometimes fatal autoimmune phenomena such as hemolytic anemias, autoimmune thrombocytopenia/thrombocytopenic purpura (ITP), Evans syndrome, and acquired hemophilia have been reported to occur in patients receiving fludarabine phosphate [see Warnings and Precautions (5.3)]. The majority of patients rechallenged with fludarabine phosphate developed a recurrence in the hemolytic process.
In post-marketing experience, cases of myelodysplastic syndrome and acute myeloid leukemia, mainly associated with prior, concomitant or subsequent treatment with alkylating agents, topoisomerase inhibitors, or irradiation have been reported.
6.2 Infections
Serious and sometimes fatal infections, including opportunistic infections and reactivations of latent viral infections such as VZV (herpes zoster), Epstein-Barr virus and JC virus (progressive multifocal leukoencephalopathy) have been reported in patients treated with fludarabine phosphate.
Rare cases of Epstein-Barr (EBV) associated lymphoproliferative disorders have been reported in patients treated with fludarabine phosphate.
In post-marketing experience, cases of progressive multifocal leukoencephalopathy have been reported. Most cases had a fatal outcome. Many of these cases were confounded by prior and/or concurrent chemotherapy. The time to onset ranged from a few weeks to approximately one year after initiating treatment.
Of the 133 adult CLL patients in the two trials, there were 29 fatalities during study, approximately 50% of which were due to infection.
6.3 Metabolic
Tumor lysis syndrome has been reported in CLL patients treated with fludarabine phosphate. This complication may include hyperuricemia, hyperphosphatemia, hypocalcemia, metabolic acidosis, hyperkalemia, hematuria, urate crystalluria, and renal failure. The onset of this syndrome may be heralded by flank pain and hematuria.
6.4 Nervous System
Objective weakness, agitation, confusion, seizures, visual disturbances, optic neuritis, optic neuropathy, blindness and coma have occurred in CLL patients treated with fludarabine phosphate at the recommended dose. Peripheral neuropathy has been observed in patients treated with fludarabine phosphate and one case of wrist-drop was reported. There have been additional reports of cerebral hemorrhage though the frequency is not known [see Warnings and Precautions (5)].
6.5 Pulmonary System
Pneumonia, a frequent manifestation of infection in CLL patients, occurred in 16%, and 22% of those treated with fludarabine phosphate in the MDAH and SWOG studies, respectively. Pulmonary hypersensitivity reactions to fludarabine phosphate characterized by dyspnea, cough and interstitial pulmonary infiltrate have been observed.
In post-marketing experience, cases of severe pulmonary toxicity have been observed with fludarabine phosphate use which resulted in ARDS, respiratory distress, pulmonary hemorrhage, pulmonary fibrosis, pneumonitis and respiratory failure. After an infectious origin has been excluded, some patients experienced symptom improvement with corticosteroids.
6.6 Gastrointestinal System
Gastrointestinal disturbances such as nausea and vomiting, anorexia, diarrhea, stomatitis, and hemorrhage have been reported in patients treated with fludarabine phosphate. Elevations of pancreatic enzyme levels have also been reported.
6.7 Cardiovascular
Edema has been frequently reported. One patient developed a pericardial effusion possibly related to treatment with fludarabine phosphate. There have been reports of heart failure and arrhythmia. No other severe cardiovascular events were considered to be drug related.
6.8 Genitourinary System
Hemorrhagic cystitis has been reported in patients treated with fludarabine phosphate.
6.9 Skin
Skin toxicity, consisting primarily of skin rashes, has been reported in patients treated with fludarabine phosphate. Erythema multiforme, Steven-Johnson syndrome, toxic epidermal necrolysis and pemphigus have been reported, with fatal outcomes in some cases.
6.10 Neoplasms
Worsening or flare-up of preexisting skin cancer lesions, as well as new onset of skin cancer, has been reported in patients during or after treatment with fludarabine phosphate.
6.12 Adverse Reactions from Clinical Trials
Data in Table 1 are derived from the 133 patients with CLL who received fludarabine phosphate in the MDAH and SWOG studies.
TABLE 1: PERCENT OF CLL PATIENTS REPORTING NON-HEMATOLOGIC ADVERSE REACTIONS
ADVERSE REACTIONS
MDAH
(N=101)
SWOG
(N=32)
ANY ADVERSE REACTION
88%
91%
BODY AS A WHOLE
72
84
FEVER
60
69
CHILLS
11
19
FATIGUE
10
38
INFECTION
33
44
PAIN
20
22
MALAISE
8
6
DIAPHORESIS
1
13
ALOPECIA
0
3
ANAPHYLAXIS
1
0
HEMORRHAGE
1
0
HYPERGLYCEMIA
1
6
DEHYDRATION
1
0
NEUROLOGICAL
21
69
WEAKNESS
9
65
PARESTHESIA
4
12
HEADACHE
3
0
VISUAL DISTURBANCE
3
15
HEARING LOSS
2
6
SLEEP DISORDER
1
3
DEPRESSION
1
0
CEREBELLAR SYNDROME
1
0
IMPAIRED MENTATION
1
0
PULMONARY
35
69
COUGH
10
44
PNEUMONIA
16
22
DYSPNEA
9
22
SINUSITIS
5
0
PHARYNGITIS
0
9
UPPER RESPIRATORY INFECTION
2
16
ALLERGIC PNEUMONITIS
0
6
EPISTAXIS
1
0
HEMOPTYSIS
1
6
BRONCHITIS
1
0
HYPOXIA
1
0
GASTROINTESTINAL
46
63
NAUSEA/VOMITING
36
31
DIARRHEA
15
13
ANOREXIA
7
34
STOMATITIS
9
0
GI BLEEDING
3
13
ESOPHAGITIS
3
0
MUCOSITIS
2
0
LIVER FAILURE
1
0
ABNORMAL LIVER FUNCTION TEST
1
3
CHOLELITHIASIS
0
3
CONSTIPATION
1
3
DYSPHAGIA
1
0
CUTANEOUS
17
18
RASH
15
15
PRURITUS
1
3
SEBORRHEA
1
0
GENITOURINARY
12
22
DYSURIA
4
3
URINARY INFECTION
2
15
HEMATURIA
2
3
RENAL FAILURE
1
0
ABNORMAL RENAL FUNCTION TEST
1
0
PROTEINURIA
1
0
HESITANCY
0
3
CARDIOVASCULAR
12
38
EDEMA
8
19
ANGINA
0
6
CONGESTIVE HEART FAILURE
0
3
ARRHYTHMIA
0
3
SUPRAVENTRICULAR TACHYCARDIA
0
3
MYOCARDIAL INFARCTION
0
3
DEEP VENOUS THROMBOSIS
1
3
PHLEBITIS
1
3
TRANSIENT ISCHEMIC ATTACK
1
0
ANEURYSM
1
0
CEREBROVASCULAR ACCIDENT
0
3
MUSCULOSKELETAL
7
16
MYALGIA
4
16
OSTEOPOROSIS
2
0
ARTHRALGIA
1
0
TUMOR LYSIS SYNDROME
1
0
More than 3000 adult patients received fludarabine phosphate in studies of other leukemias, lymphomas, and other solid tumors. The spectrum of adverse effects reported in these studies was consistent with the data presented above.
- 7 DRUG INTREACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
[See Warnings and Precautions (5.6)].
Based on its mechanism of action, fludarabine phosphate can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of Fludarabine Phosphate Injection in pregnant women. In rats, repeated intravenous doses of fludarabine phosphate at 2.4 times and 7.2 times the recommended human intravenous dose (25 mg/m 2) administered during organogenesis caused an increase in resorptions, skeletal and visceral malformations (cleft palate, exencephaly, and fetal vertebrae deformities) and decreased fetal body weights. Maternal toxicity was not apparent at 2.4 times the human intravenous dose, and was limited to slight body weight decreases at 7.2 times the human intravenous dose. In rabbits, repeated intravenous doses of fludarabine phosphate at 3.8 times the human intravenous dose administered during organogenesis increased embryo and fetal lethality as indicated by increased resorptions and a decrease in live fetuses. A significant increase in malformations including cleft palate, hydrocephaly, adactyly, brachydactyly, fusions of the digits, diaphragmatic hernia, heart/great vessel defects, and vertebrae/rib anomalies were seen in all dose levels (≥ 0.5 times the human intravenous dose). If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
8.3 Nursing Mothers
It is not known whether fludarabine phosphate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions including tumorigenicity in nursing infants, a decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug for the mother.
8.4 Pediatric Use
Data submitted to the FDA was insufficient to establish efficacy in any childhood malignancy.
Fludarabine phosphate was evaluated in 62 pediatric patients (median age 10, range 1 to 21) with refractory acute leukemia (45 patients) or solid tumors (17 patients). Limited pharmacokinetic data for fludarabine phosphate are available in children (ages 1 to 21 years). When fludarabine phosphate was administered as a loading dose over 10 minutes immediately followed by a 5-day continuous infusion, steady-state conditions were reached early.
The fludarabine phosphate regimen tested for pediatric lymphocytic leukemia (ALL) patients was a loading bolus of 10.5 mg/m 2/day followed by a continuous infusion of 30.5 mg/m 2/day for 5 days. In 12 pediatric patients with solid tumors, dose-limiting myelosuppression was observed with a loading dose of 8 mg/m 2/day followed by a continuous infusion of 23.5 mg/m 2/day for 5 days. The maximum tolerated dose was a loading dose of 7 mg/m 2/day followed by a continuous infusion of 20 mg/m 2/day for 5 days. Treatment toxicity included bone marrow suppression. Platelet counts appeared to be more sensitive to the effects of fludarabine phosphate than hemoglobin and white blood cell counts. Other adverse events included fever, chills, asthenia, rash, nausea, vomiting, diarrhea, and infection. There were no reported occurrences of peripheral neuropathy or pulmonary hypersensitivity reaction.
8.6 Patients with Renal Impairment
The total body clearance of the principal metabolite 2-fluoro-ara-A correlated with the creatinine clearance, indicating the importance of the renal excretion pathway for the elimination of the drug. Renal clearance represents approximately 40% of the total body clearance. Patients with creatinine clearance 30 to 79 mL/min should have their fludarabine phosphate dose reduced and be monitored closely for excessive toxicity. Due to insufficient data, fludarabine phosphate should not be administered to patients with creatinine clearance less than 30 mL/min [see Dosage and Administration (2.2), Warnings and Precautions (5.9)].
-
10 OVERDOSAGE
High doses of fludarabine phosphate [see Warnings and Precautions (5)] have been associated with an irreversible central nervous system toxicity characterized by delayed blindness, coma and death. High doses are also associated with severe thrombocytopenia and neutropenia due to bone marrow suppression. There is no known specific antidote for fludarabine phosphate overdosage. Treatment consists of drug discontinuation and supportive therapy.
-
11 DESCRIPTION
Fludarabine Phosphate Injection contains fludarabine phosphate, a nucleotide metabolic inhibitor. Fludarabine phosphate is a fluorinated nucleotide analog of the antiviral agent vidarabine, 9- ß-D-arabinofuranosyladenine (ara-A), that is relatively resistant to deamination by adenosine deaminase.
The chemical name for fludarabine phosphate is 9H-Purin-6-amine, 2-fluoro-9-(5-0- phosphono- ß-D-arabinofuranosyl)(2-fluoro-ara-AMP). The molecular formula of fludarabine phosphate is C 10H 13FN 5O 7P (MW 365.2) and the structure is provided in Figure 1.
Figure 1: Chemical Structure of Fludarabine Phosphate
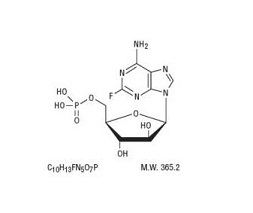
Each mL contains 25 mg of the active ingredient fludarabine phosphate, 25 mg of mannitol, water for injection, q.s.; and sodium hydroxide to adjust pH to 6.8. The pH range for the final product is 6.0 to 7.1. Fludarabine Phosphate Injection is a sterile solution intended for intravenous administration.
-
12 CLINICAL PHARMACOLOGY
12 Mechanism of Action
Fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. This metabolite appears to act by inhibiting DNA polymerase alpha, ribonucleotide reductase and DNA primase, thus inhibiting DNA synthesis. The mechanism of action of this antimetabolite is not completely characterized and may be multi-faceted.
12.3 Pharmacokinetics
Phase I studies in humans have demonstrated that fludarabine phosphate is rapidly converted to the active metabolite, 2-fluoro-ara-A, within minutes after intravenous infusion. Consequently, clinical pharmacology studies have focused on 2-fluoro-ara-A pharmacokinetics. After the five daily doses of 25 mg 2-fluoro-ara-AMP/m2 to cancer patients infused over 30 minutes, 2-fluoro-ara-A concentrations show a moderate accumulation. During a 5-day treatment schedule, 2-fluoroara-A plasma trough levels increased by a factor of about 2. The terminal half-life of 2-fluoro-ara-A was estimated as approximately 20 hours. In vitro, plasma protein binding of fludarabine ranged between 19% and 29%. A correlation was noted between the degree of absolute granulocyte count nadir and increased area under the concentration x time curve (AUC).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal carcinogenicity studies with fludarabine have been conducted.
Fludarabine phosphate was clastogenic in vitro to Chinese hamster ovary cells (chromosome aberrations in the presence of metabolic activation) and induced sister chromatid exchanges both with and without metabolic activation. In addition, fludarabine phosphate was clastogenic in vivo (mouse micronucleus assay) but was not mutagenic to germ cells (dominant lethal test in male mice). Fludarabine phosphate was not mutagenic to bacteria (Ames test) or mammalian cells (HGRPT assay in Chinese hamster ovary cells) either in the presence or absence of metabolic activation.
Studies in mice, rats and dogs have demonstrated dose-related adverse effects on the male reproductive system. Observations consisted of a decrease in mean testicular weights in mice and rats with a trend toward decreased testicular weights in dogs and degeneration and necrosis of spermatogenic epithelium of the testes in mice, rats and dogs. The possible adverse effects on fertility in humans have not been adequately evaluated [see Warnings and Precautions (5.7)].
-
14 CLINICAL STUDIES
14.1 Adults
Two single-arm open-label studies of fludarabine phosphate have been conducted in adult patients with CLL refractory to at least one prior standard alkylating-agent containing regimen. In a study conducted by M.D. Anderson Cancer Center (MDAH), 48 patients were treated with a dose of 22 to 40 mg/m 2 daily for 5 days every 28 days. Another study conducted by the Southwest Oncology Group (SWOG) involved 31 patients treated with a dose of 15 to 25 mg/m 2 daily for 5 days every 28 days. The overall objective response rates were 48% and 32% in the MDAH and SWOG studies, respectively. The complete response rate in both studies was 13%; the partial response rate was 35% in the MDAH study and 19% in the SWOG study. These response rates were obtained using standardized response criteria developed by the National Cancer Institute CLL Working Group and were achieved in heavily pretreated patients. The ability of fludarabine phosphate to induce a significant rate of response in refractory patients suggests minimal cross-resistance with commonly used anti-CLL agents.
The median time to response in the MDAH and SWOG studies was 7 weeks (range of 1 to 68 weeks) and 21 weeks (range of 1 to 53 weeks), respectively. The median duration of disease control was 91 weeks (MDAH) and 65 weeks (SWOG). The median survival of all refractory CLL patients treated with fludarabine phosphate was 43 weeks and 52 weeks in the MDAH and SWOG studies, respectively.
Rai stage improved to Stage II or better in 7 of 12 MDAH responders (58%) and in 5 of 7 SWOG responders (71%) who were Stage III or IV at baseline. In the combined studies, mean hemoglobin concentration improved from 9.0 g/dL at baseline to 11.8 g/dL at the time of response in a subgroup of anemic patients. Similarly, average platelet count improved from 63,500/mm 3 to 103,300/mm 3 at the time of response in a subgroup of patients who were thrombocytopenic at baseline.
-
15 REFERENCES
- Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. NIOSH Alert 2004-165.
- OSHA Technical Manual, TED 1-0.l5A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otim_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs.
Am J Health-Syst Pharm. 2006; 63:172-1193.
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Fludarabine Phosphate Injection, USP is supplied as a sterile solution containing 50 mg/2 mL (25 mg/mL) of fludarabine phosphate in a 2 mL single use vial.
NDC: 59923-604-02 one carton containing 1 vial of Fludarabine Phosphate Injection.
16.3 Handling and Disposal
Procedures for proper handling and disposal should be considered. Consideration should be given to handling and disposal according to guidelines issued for cytotoxic drugs. Several guidelines on this subject have been published. 1-4 Caution should be exercised in the handling and preparation of Fludarabine Phosphate Injection solution. The use of latex gloves and safety glasses is recommended to avoid exposure in case of breakage of the vial or other accidental spillage. If the solution contacts the skin or mucous membranes, wash thoroughly with soap and water; rinse eyes thoroughly with plain water. Avoid exposure by inhalation or by direct contact of the skin or mucous membranes.
-
17 PATIENT COUNSELING INFORMATION
17.1 Monitoring
Patients should be informed of the importance of periodic assessment of their blood count to detect the development of anemia, neutropenia and thrombocytopenia.
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
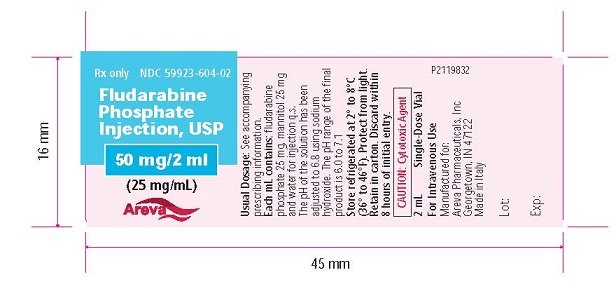
Principal Display Panel - Vial
RX only
NDC: 59923-604-02
Fludarabine Phosphate Injection, USP
50 mg/2 mL
(25 mg/mL)
Areva
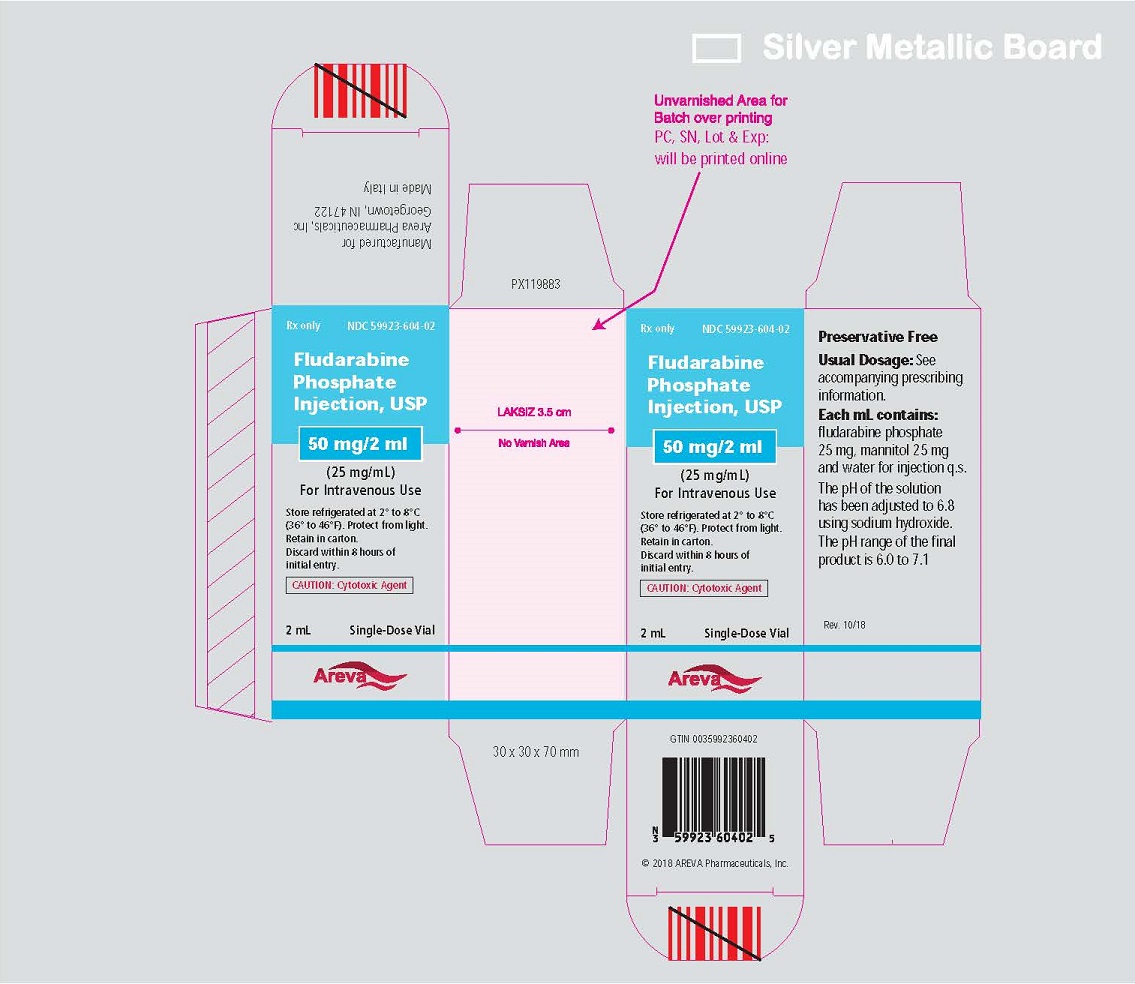
Principal Display Panel - Carton
RX only
NDC: 59923-604-02
Fludarabine Phosphate Injection, USP
50 mg/2 mL
(25 mg/mL)
For Intravenous Use
Store refrigerated at 2° and 8°C (36° and 46°F)
Protect from light. Retain in carton.
Discard within 8 hours of initial entry.
CAUTION: CYTOTOXIC AGENT
2 mL Single-Dose Vial
Areva
-
INGREDIENTS AND APPEARANCE
FLUDARABINE PHOSPHATE
fludarabine phosphate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59923-604 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUDARABINE PHOSPHATE (UNII: 1X9VK9O1SC) (FLUDARABINE - UNII:P2K93U8740) FLUDARABINE PHOSPHATE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) MANNITOL (UNII: 3OWL53L36A) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59923-604-02 1 in 1 CARTON 12/31/2019 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090724 12/31/2019 Labeler - Areva Pharmaceuticals (833189835) Registrant - Areva Pharmaceuticals (833189835) Establishment Name Address ID/FEI Business Operations NerPharMa SRL 338839192 manufacture(59923-604)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.