XIAFLEX- collagenase clostridium histolyticum kit
XIAFLEX by
Drug Labeling and Warnings
XIAFLEX by is a Prescription medication manufactured, distributed, or labeled by Endo Pharmaceuticals Inc., Auxilium Pharmaceuticals, LLC, Charles River Biopharmaceutical Services GmbH, Eurofins Lancaster Laboratories, Inc., Jubilant HollisterStier LLC, KBI BioPharma, Inc., Nelson Laboratories, Inc., Packaging Coordinators, Inc., Patheon Manufacturing Services LLC, West Pharmaceutical Services, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XIAFLEX safely and effectively. See full prescribing information for XIAFLEX.
XIAFLEX® (collagenase clostridium histolyticum) for injection, for intralesional use
Initial U.S. Approval: 2010WARNING: CORPORAL RUPTURE (PENILE FRACTURE) OR OTHER SERIOUS PENILE INJURY IN THE TREATMENT OF PEYRONIE’S DISEASE
See full prescribing information for complete boxed warning
- Corporal rupture (penile fracture) was reported as an adverse reaction in 5 of 1044 (0.5%) XIAFLEX-treated patients in clinical studies. In other XIAFLEX-treated patients (9 of 1044; 0.9%), a diagnosis of corporal rupture cannot be excluded. Severe penile hematoma was also reported as an adverse reaction in 39 of 1044 (3.7%) XIAFLEX-treated patients (5.2).
- XIAFLEX is available for the treatment of Peyronie’s disease only through a restricted program called the XIAFLEX REMS Program (5.3).
INDICATIONS AND USAGE
XIAFLEX is a combination of bacterial collagenases indicated for:
DOSAGE AND ADMINISTRATION
Dupuytren’s Contracture (2.1)
- XIAFLEX should be administered by a healthcare provider experienced in injection procedures of the hand and in the treatment of Dupuytren’s contracture.
- Reconstitute XIAFLEX lyophilized powder with only the supplied diluent prior to use.
- Inject 0.58 mg of XIAFLEX into each palpable Dupuytren’s cord with a contracture of a metacarpophalangeal (MP) joint or a proximal interphalangeal (PIP) joint according to the injection procedure.
- Up to two joints in the same hand may be treated during a treatment visit (2.1).
- Approximately 24 to 72 hours following an injection, perform a finger extension procedure if a contracture persists.
- Injections and finger extension procedures may be administered up to 3 times per cord at approximately 4-week intervals.
- Inject up to two cords in the same hand at a treatment visit. If a patient has other cords with contractures, inject those cords at another treatment visit.
Peyronie’s Disease (2.2)
- XIAFLEX should be administered by a healthcare provider experienced in the treatment of male urological diseases.
- Reconstitute XIAFLEX lyophilized powder with only the supplied diluent prior to use.
- A treatment cycle consists of two XIAFLEX injection procedures and a penile modeling procedure.
- Induce a penile erection. A single intracavernosal injection of 10 or 20 micrograms of alprostadil may be used for this purpose.
- With the penis in the erect state, identify and mark the target area in the Peyronie’s plaque to be injected.
- The penis should be in a flaccid state before injecting XIAFLEX.
- Inject 0.58 mg XIAFLEX into the target plaque once on each of two days, 1 to 3 days apart, according to the injection procedure.
- Perform a penile modeling procedure 1 to 3 days after the second injection of each treatment cycle.
- For each plaque causing the curvature deformity, up to four treatment cycles may be administered. Each treatment cycle may be repeated at approximately six-week intervals. If the curvature deformity is less than 15 degrees after the first, second or third treatment cycle, or if further treatment is not clinically indicated, then subsequent treatment cycles should not be administered.
DOSAGE FORMS AND STRENGTHS
Single-use glass vials containing 0.9 mg of collagenase clostridium histolyticum as a sterile, lyophilized powder for reconstitution. Sterile diluent for reconstitution is also provided in a single-use glass vial. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Tendon rupture or serious injury to the injected finger/hand: Avoid injecting XIAFLEX into tendons, nerves, blood vessels, or other collagen-containing structure of the hand. Injection into these structures may result in possible permanent injury, such as tendon rupture, ligament damage, or skin laceration. (5.1)
- Corporal rupture (penile fracture) or other serious injury to the penis: Avoid injecting into the urethra, nerves, blood vessels, corpora cavernosa or other collagen-containing structures of the penis. Injection into these structures may result in possible permanent injury such as corporal rupture (penile fracture). (5.2)
- Hypersensitivity reactions, including anaphylaxis: Healthcare providers should be prepared to address hypersensitivity reactions, including anaphylaxis, following XIAFLEX injections. (5.4)
- Patients with abnormal coagulation: Use with caution, including in patients who have received anticoagulant medications other than low-dose aspirin within 7 days of the injection. (5.5)
ADVERSE REACTIONS
Dupuytren’s Contracture (6.1)
The most common adverse reactions reported in ≥ 25% of patients treated with XIAFLEX and at an incidence greater than placebo were edema peripheral (e.g., swelling of the injected hand), contusion, injection site hemorrhage, injection site reaction, and pain in the injected extremity.
Peyronie’s Disease (6.2)
The most frequently reported adverse drug reactions reported with ≥ 25% of patients treated with XIAFLEX and at an incidence greater than placebo were penile hematoma, penile swelling and penile pain.
To report SUSPECTED ADVERSE REACTIONS, contact Endo Pharmaceuticals, Inc. at 1-800-462-3636 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CORPORAL RUPTURE (PENILE FRACTURE) OR OTHER SERIOUS PENILE INJURY IN THE TREATMENT OF PEYRONIE’S DISEASE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration for Dupuytren’s Contracture
2.2 Dosage and Administration for Peyronie’s Disease
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Tendon Rupture or Other Serious Injury to the Injected Finger/Hand in the Treatment of Dupuytren’s Contracture
5.2 Corporal Rupture (Penile Fracture) or Other Serious Injury to the Penis in the Treatment of Peyronie’s Disease
5.3 XIAFLEX REMS Program
5.4 Hypersensitivity Reactions, Including Anaphylaxis
5.5 Risk of Bleeding in Patients with Abnormal Coagulation
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience in Patients with Dupuytren’s Contracture
6.2 Clinical Studies Experience in Patients with Peyronie’s Disease
6.3 Immunogenicity
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Dupuytren’s Contracture
14.2 Peyronie’s Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Patient Counseling for Dupuytren’s Contracture
17.2 Patient Counseling for Peyronie’s Disease
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CORPORAL RUPTURE (PENILE FRACTURE) OR OTHER SERIOUS PENILE INJURY IN THE TREATMENT OF PEYRONIE’S DISEASE
Corporal rupture (penile fracture) was reported as an adverse reaction in 5 of 1044 (0.5%) XIAFLEX-treated patients in clinical studies. In other XIAFLEX-treated patients (9 of 1044; 0.9%), a combination of penile ecchymoses or hematoma, sudden penile detumescence, and/or a penile “popping” sound or sensation was reported, and in these cases, a diagnosis of corporal rupture cannot be excluded. Severe penile hematoma was also reported as an adverse reaction in 39 of 1044 (3.7%) XIAFLEX-treated patients [see Warnings and Precautions (5.2)].
Signs or symptoms that may reflect serious penile injury should be promptly evaluated to assess for corporal rupture or severe penile hematoma which may require surgical intervention [see Warnings and Precautions (5.2)].
Because of the risks of corporal rupture or other serious penile injury, XIAFLEX is available for the treatment of Peyronie’s disease only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the XIAFLEX REMS Program [see Warnings and Precautions (5.3)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration for Dupuytren’s Contracture
Dosing Overview for Dupuytren’s Contracture
XIAFLEX should be administered by a healthcare provider experienced in injection procedures of the hand and in the treatment of patients with Dupuytren’s contracture.
XIAFLEX, supplied as a lyophilized powder, must be reconstituted with the provided diluent prior to use [see Dosage and Administration (2.1)]. The dose of XIAFLEX is 0.58 mg per injection into a palpable cord with a contracture of a metacarpophalangeal (MP) joint or a proximal interphalangeal (PIP) joint [see Dosage and Administration (2.1)]. Each vial of XIAFLEX and sterile diluent should only be used for a single injection. If two joints on the same hand are to be treated during a treatment visit, separate vials and syringes should be used for each reconstitution and injection.
Table 1 displays an overview of the volumes of sterile diluent for reconstitution and the reconstituted XIAFLEX solution to be used in the intralesional injection [see Dosage and Administration (2.1)]. Approximately 24 to 72 hours after injection, perform a finger extension procedure if a contracture persists to facilitate cord disruption [see Dosage and Administration (2.1)].
Table 1. Volumes Needed for Reconstitution and Administration for Dupuytren’s Contracture For cords affecting
MP jointsFor cords affecting
PIP joints1 The reconstituted XIAFLEX solution to be used in the intralesional injection contains 0.58 mg of XIAFLEX.
Note: The entire reconstituted XIAFLEX solution contains 0.9 mg of XIAFLEX.
Reconstituted XIAFLEX solution remaining in the vial after the injection should be discarded.Sterile Diluent for Reconstitution Volume 0.39 mL 0.31 mL Reconstituted XIAFLEX Solution to be Injected1 Volume 0.25 mL 0.20 mL Four weeks after the XIAFLEX injection and finger extension procedure, if a MP or PIP contracture remains, the cord may be re-injected with a single dose of 0.58 mg of XIAFLEX and the finger extension procedure may be repeated (approximately 24 to 72 hours after injection). Injections and finger extension procedures may be administered up to 3 times per cord at approximately 4-week intervals.
Perform up to two injections in the same hand according to the injection procedure during a treatment visit. Two palpable cords affecting two joints may be injected or one palpable cord affecting two joints in the same finger may be injected at two locations during a treatment visit. If patient has other palpable cords with contractures of MP or PIP joints, these cords may be injected with XIAFLEX at other treatment visits approximately 4 weeks apart.
Reconstitution of the Lyophilized Powder for Dupuytren’s Contracture
- Before use, remove the vial(s) containing the lyophilized powder of XIAFLEX and the vial(s) containing the diluent for reconstitution from the refrigerator and allow the vials to stand at room temperature for at least 15 minutes and no longer than 60 minutes. Visually inspect the vial(s) containing XIAFLEX. The cake of lyophilized powder should be intact and white in color.
- After removal of the flip-off cap from each vial, using aseptic technique swab the rubber stopper and surrounding surface of the vial(s) containing XIAFLEX and the vial(s) containing the diluent for reconstitution with sterile alcohol (no other antiseptics should be used).
- Use only the supplied diluent for reconstitution. The diluent contains calcium which is required for the activity of XIAFLEX.
- Using a 1 mL syringe that contains 0.01 mL graduations with a 27-gauge ½-inch needle (not supplied), withdraw a volume of the diluent supplied, as follows:
- 0.39 mL for cords affecting a MP joint or
-
0.31 mL for cords affecting a PIP joint.
- Inject the diluent slowly into the sides of the vial containing the lyophilized powder of XIAFLEX. Do not invert the vial or shake the solution. Slowly swirl the solution to ensure that all of the lyophilized powder has gone into solution. If administering two injections in the same hand during a treatment visit, use a new syringe to reconstitute a second vial of XIAFLEX with a second vial of diluent.
- The reconstituted XIAFLEX solution can be kept at room temperature (20° to 25°C/68° to 77°F) for up to one hour or refrigerated at 2° to 8°C (36° to 46°F) for up to 4 hours prior to administration. If the reconstituted XIAFLEX solution is refrigerated, allow this solution to return to room temperature for approximately 15 minutes before use.
- Discard the syringe(s) and needle(s) used for reconstitution and the diluent vial(s).
Preparation Prior to Injection for Dupuytren’s Contracture
- The reconstituted XIAFLEX solution should be clear. Inspect the solution visually for particulate matter and discoloration prior to administration. If the solution contains particulates, is cloudy, or is discolored, do not inject the reconstituted solution.
- Administration of a local anesthetic agent prior to injection is not recommended, as it may interfere with proper placement of the XIAFLEX injection.
- If injecting into a cord affecting the PIP joint of the fifth finger, care should be taken to inject as close to the palmar digital crease as possible (as far proximal to the digital PIP joint crease), and the needle insertion should not be more than 2 to 3 mm in depth. Tendon ruptures occurred after XIAFLEX injections near the digital PIP joint crease [see Warnings and Precautions (5.1)].
- Reconfirm the cord(s) to be injected. The site chosen for each injection should be the area where the contracting cord is maximally separated from the underlying flexor tendons and where the skin is not intimately adhered to the cord.
- Apply an antiseptic at the site(s) of the injection(s) and allow the skin to dry.
Injection Procedure for Dupuytren’s Contracture
- Using a new 1 mL hubless syringe that contains 0.01 mL graduations with a permanently fixed, 27-gauge ½-inch needle (not supplied), withdraw a volume of reconstituted solution (containing 0.58 mg of XIAFLEX) as follows:
- 0.25 mL for cords affecting a MP joint or
-
0.20 mL for cords affecting a PIP joint.
- With your non-dominant hand, secure the patient’s hand to be treated while simultaneously applying tension to the cord. With your dominant hand, place the needle into the cord, using caution to keep the needle within the cord. Avoid having the needle tip pass completely through the cord to help minimize the potential for injection of XIAFLEX into tissues other than the cord [see Warnings and Precautions (5.1)]. After needle placement, if there is any concern that the needle is in the flexor tendon, apply a small amount of passive motion at the distal interphalangeal (DIP) joint. If insertion of the needle into a tendon is suspected or paresthesia is noted by the patient, withdraw the needle and reposition it into the cord.
- If the needle is in the proper location, there will be some resistance noted during the injection procedure. After confirming that the needle is correctly placed in the cord, inject approximately one-third of the dose.
- Next, withdraw the needle tip from the cord and reposition it in a slightly more distal location (approximately 2 to 3 mm) to the initial injection in the cord and inject another one-third of the dose.
- Again withdraw the needle tip from the cord and reposition it a third time proximal to the initial injection (approximately 2 to 3 mm) and inject the final portion of the dose into the cord.
- When administering two injections in the same hand during a treatment visit, use a new syringe and separate vial of reconstituted solution for each injection. Repeat steps a through f.
- When administering two injections in the same hand during a treatment visit, begin with the affected finger in the most medial aspect of the hand and continue toward the lateral aspect (eg, fifth finger to index finger). When administering two injections in a cord affecting two joints in the same finger, begin with the affected joint in the most proximal aspect of the finger and continue toward the distal aspect (eg, MP to PIP).
- Wrap the patient’s treated hand with a soft, bulky, gauze dressing.
- Instruct the patient to limit motion of the treated finger(s) and to keep the injected hand elevated until bedtime.
- Instruct the patient not to attempt to disrupt the injected cord(s) by self-manipulation and to return to the healthcare provider’s office the next day for follow-up and a finger extension procedure(s), if needed.
- Discard the unused portion of the reconstituted solution and diluent after injection. Do not store, pool, or use any vials containing unused reconstituted solution or diluent.
Finger Extension Procedure for Dupuytren’s Contracture
- At the follow-up visit approximately 24 to 72 hours after the injection(s), if a contracture remains, perform a passive finger extension procedure on each treated joint (as described below) to facilitate cord disruption. If two joints in one finger were treated, perform the finger extension procedure on the affected MP joint before performing the finger extension procedure on the affected PIP joint.
- Local anesthesia may be used. Avoid direct pressure on the injection site as it will likely be tender. Care should be taken during release of contracture, as some patients may experience skin splitting. If this occurs, cover the area with gauze and apply gentle pressure until bleeding stops. Standard wound care with regular dressings should be applied.
- While the patient’s wrist is in the flexed position, apply moderate stretching pressure to the injected cord by extending the finger for approximately 10 to 20 seconds. For cords affecting the PIP joint, perform the finger extension procedure when the MP joint is in the flexed position.
- If the first finger extension procedure does not result in disruption of the cord, a second and third attempt can be performed at 5- to 10-minute intervals. However, no more than 3 attempts per joint are recommended to disrupt a cord.
- If the cord has not been disrupted after 3 attempts, a follow-up visit may be scheduled in approximately 4 weeks. If, at that subsequent visit, the contracted cord persists, an additional XIAFLEX injection with finger extension procedures may be performed [see Dosage and Administration (2.1)].
- Following the finger extension procedure(s), fit patient with a splint and provide instructions for use at bedtime for up to 4 months to maintain finger extension. Also, instruct the patient to perform finger extension and flexion exercises several times a day for several months.
2.2 Dosage and Administration for Peyronie’s Disease
Dosing Overview for Peyronie's Disease
XIAFLEX should be administered by a healthcare provider experienced in the treatment of male urological diseases, who has completed required training for use of XIAFLEX in the treatment of Peyronie’s disease.XIAFLEX, supplied as lyophilized powder, must be reconstituted with the provided diluent prior to use [see Dosage and Administration (2.2)]. The dose of XIAFLEX is 0.58 mg per injection administered into a Peyronie’s plaque. If more than one plaque is present, inject into the plaque causing the curvature deformity.
A treatment course consists of a maximum of 4 treatment cycles. Each treatment cycle consists of two XIAFLEX injection procedures [see Dosage and Administration (2.2)] and one penile modeling procedure [see Dosage and Administration (2.2)]. The second XIAFLEX injection procedure is performed 1 to 3 days after the first. The penile modeling procedure is performed 1 to 3 days after the second injection of the treatment cycle. The interval between treatment cycles is approximately six weeks. The treatment course therefore, consists of a maximum of 8 injection procedures and 4 modeling procedures.
If the curvature deformity is less than 15 degrees after the first, second or third treatment cycle, or if the healthcare provider determines that further treatment is not clinically indicated, then the subsequent treatment cycles should not be administered.
The safety of more than one treatment course of XIAFLEX is not known.
Table 2 displays an overview of the volume of sterile diluent for reconstitution and the reconstituted XIAFLEX solution to be used in the intralesional injection [see Dosage and Administration (2.2)].
Table 2. Volumes Needed for Reconstitution and Administration 1 The reconstituted XIAFLEX solution to be used in the intralesional injection contains 0.58 mg of XIAFLEX. Note: The entire reconstituted XIAFLEX solution contains 0.9 mg of XIAFLEX.
Reconstituted XIAFLEX solution remaining in the vial after the injection should be discarded.Sterile Diluent for Reconstitution Volume 0.39 mL Reconstituted XIAFLEX Solution to be Injected1 Volume 0.25 mL Reconstitution of the Lyophilized Powder for Peyronie’s Disease
- Before use, remove the vial containing the lyophilized powder of XIAFLEX and the vial containing the diluent for reconstitution from the refrigerator and allow the two vials to stand at room temperature for at least 15 minutes and no longer than 60 minutes. Visually inspect the vial containing XIAFLEX. The cake of lyophilized powder should be intact and white in color.
- After removal of the flip-off cap from each vial, using aseptic technique swab the rubber stopper and surrounding surface of the vial containing XIAFLEX and the vial containing the diluent for reconstitution with sterile alcohol (no other antiseptics should be used).
- Use only the supplied diluent for reconstitution. The diluent contains calcium which is required for the activity of XIAFLEX.
- Using a 1 mL syringe with 0.01 mL graduations with a 27-gauge ½-inch needle (not supplied), withdraw a volume of 0.39 mL of the diluent supplied.
- Inject the diluent slowly into the sides of the vial containing the lyophilized powder of XIAFLEX. Do not invert the vial or shake the solution. Slowly swirl the solution to ensure that all of the lyophilized powder has gone into solution.
- The reconstituted XIAFLEX solution can be kept at room temperature (20° to 25°C/68° to 77°F) for up to one hour or refrigerated at 2° to 8°C (36° to 46°F) for up to 4 hours prior to administration. If the reconstituted XIAFLEX solution is refrigerated, allow this solution to return to room temperature for approximately 15 minutes before use.
- Discard the syringe and needle used for reconstitution and the diluent vial.
Identification of Treatment Area for Peyronie’s Disease
- Prior to each treatment cycle, identify the treatment area as follows:
- Induce a penile erection. A single intracavernosal injection of 10 or 20 micrograms of alprostadil may be used for this purpose. Apply antiseptic at the site of the injection and allow the skin to dry prior to the intracavernosal injection.
- Locate the plaque at the point of maximum concavity (or focal point) in the bend of the penis.
- Mark the point with a surgical marker. This indicates the target area in the plaque for XIAFLEX deposition.
Injection Procedure for Peyronie’s Disease
- The reconstituted XIAFLEX solution should be clear. Inspect the solution visually for particulate matter and discoloration prior to administration. If the solution contains particulates, is cloudy, or is discolored, do not inject the reconstituted solution.
- Apply antiseptic at the site of the injection and allow the skin to dry.
- Administer suitable local anesthetic, if desired.
- Using a new hubless syringe containing 0.01 mL graduations with a permanently fixed 27-gauge ½-inch needle (not supplied), withdraw a volume of 0.25 mL of reconstituted solution (containing 0.58 mg of XIAFLEX).
- The penis should be in a flaccid state before XIAFLEX is injected. Place the needle tip on the side of the target plaque in alignment with the point of maximal concavity. Orient the needle so that it enters the edge of the plaque and advance the needle into the plaque itself from the side. Do not advance the needle beneath the plaque nor perpendicularly towards the corpora cavernosum.
- Insert and advance the needle transversely through the width of the plaque, towards the opposite side of the plaque without passing completely through it. Proper needle position is tested and confirmed by carefully noting resistance to minimal depression of the syringe plunger.
- With the tip of the needle placed within the plaque, initiate injection, maintaining steady pressure to slowly inject XIAFLEX into the plaque. Withdraw the needle slowly so as to deposit the full dose along the needle track within the plaque. For plaques that are only a few millimeters in width, the distance of withdrawal of the syringe may be very minimal. The goal is always to deposit the full dose entirely within the plaque.
- Upon complete withdrawal of the needle, apply gentle pressure at the injection site. Apply a dressing as necessary.
- Discard the unused portion of the reconstituted solution and diluent after each injection. Do not store, pool, or use any vials containing unused reconstituted solution or diluent.
- The second injection of each treatment cycle should be made approximately 2 to 3 mm apart from the first injection.
Penile Modeling Procedure for Peyronie’s Disease
Penile modeling helps relieve curvature deformity and straighten the penile shaft. At a follow-up visit 1 to 3 days after the second injection of each treatment cycle, perform a penile modeling procedure (as described below) on the flaccid penis to stretch and elongate the treated plaque:
- Administer suitable local anesthetic, if desired.
- Wearing gloves, grasp the plaque or indurated portion of the flaccid penis about 1 cm proximal and distal to the injection site. Avoid direct pressure on the injection site.
- Using the target plaque as a fulcrum point, use both hands to apply firm, steady pressure to elongate and stretch the plaque. The goal is to gradually create bending opposite to the patient’s penile curvature, with stretching to the point of moderate resistance. Hold pressure for 30 seconds then release.
- After a 30 second rest period, repeat the penile modeling technique for a total of 3 modeling attempts at 30 seconds for each attempt.
In addition to the in-office penile modeling procedure, patients should be instructed to self-perform penile modeling activities at home each day for the 6-week period following the investigator penile plaque modeling visit of each treatment cycle as follows:
- During spontaneous erections, gently attempt to straighten the penis without producing pain and hold the penis in a straightened position for 30 seconds.
- The flaccid penis should be gently stretched three times daily. Slow, gentle force should be used without producing pain.
-
3 DOSAGE FORMS AND STRENGTHS
XIAFLEX is supplied in single-use glass vials containing 0.9 mg of collagenase clostridium histolyticum as a sterile, lyophilized powder for reconstitution. Sterile diluent for reconstitution is provided in the package in a single-use glass vial containing 3 mL of 0.3 mg/mL calcium chloride dihydrate in 0.9% sodium chloride.
-
4 CONTRAINDICATIONS
XIAFLEX is contraindicated in:
- the treatment of Peyronie’s plaques that involve the penile urethra due to potential risk to this structure.
- patients with a history of hypersensitivity to XIAFLEX or to collagenase used in any other therapeutic application or application method [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Tendon Rupture or Other Serious Injury to the Injected Finger/Hand in the Treatment of Dupuytren’s Contracture
In the controlled and uncontrolled portions of clinical trials in Dupuytren’s contracture, flexor tendon ruptures occurred after XIAFLEX injection [see Adverse Reactions (6.1)]. Injection of XIAFLEX into collagen-containing structures such as tendons or ligaments of the hand may result in damage to those structures and possible permanent injury such as tendon rupture or ligament damage. Therefore, XIAFLEX should be injected only into the collagen cord with a MP or PIP joint contracture, and care should be taken to avoid injecting into tendons, nerves, blood vessels, or other collagen-containing structures of the hand. When injecting a cord affecting a PIP joint of the fifth finger, the needle insertion should not be more than 2 to 3 mm in depth and avoid injecting more than 4 mm distal to the palmar digital crease [see Dosage and Administration (2.1)].
Other XIAFLEX-associated serious local adverse reactions included pulley rupture, ligament injury, complex regional pain syndrome (CRPS), sensory abnormality of the hand, and skin laceration (tear). In a historically controlled post-marketing trial, the incidence of skin laceration (22%) was higher for subjects treated with two concurrent injections of XIAFLEX compared with subjects treated with up to three single injections in the placebo-controlled premarketing trials (9%). Cases of skin laceration requiring skin graft after finger extension procedures have been reported post-marketing. Signs or symptoms that may reflect serious injury to the injected finger/hand should be promptly evaluated because surgical intervention may be required.
5.2 Corporal Rupture (Penile Fracture) or Other Serious Injury to the Penis in the Treatment of Peyronie’s Disease
Corporal rupture was reported as an adverse reaction after XIAFLEX injections in 5 of 1044 (0.5%) XIAFLEX treated patients in the controlled and uncontrolled clinical trials in Peyronie’s disease.
In other XIAFLEX-treated patients (9 of 1044; 0.9%), a combination of penile ecchymoses or hematoma, sudden penile detumescence, and/or a penile “popping” sound or sensation was reported, and in these cases, a diagnosis of corporal rupture can not be excluded. These patients were managed without surgical intervention, but the long-term consequences are unknown.
Severe penile hematoma was also reported as an adverse reaction in 39 of 1044 patients (3.7%) in the controlled and uncontrolled clinical trials in Peyronie’s disease [see Adverse Reactions (6)].
Signs or symptoms that may reflect serious injury to the penis should be promptly evaluated in order to assess for corporal rupture or severe penile hematoma, which may require surgical intervention.
Injection of XIAFLEX into collagen-containing structures such as the corpora cavernosa of the penis may result in damage to those structures and possible injury such as corporal rupture (penile fracture). Therefore, XIAFLEX should be injected only into the Peyronie’s plaque and care should be taken to avoid injecting into the urethra, nerves, blood vessels, corpora cavernosa or other collagen-containing structures of the penis.
5.3 XIAFLEX REMS Program
Because of the risks of corporal rupture (penile fracture) or other serious penile injury in the treatment of Peyronie’s disease, XIAFLEX is available only through the XIAFLEX REMS Program [see Warnings and Precautions (5.2)].
Required components of the XIAFLEX REMS Program include the following:
- Prescribers must be certified with the program by enrolling and completing training in the administration of XIAFLEX treatment for Peyronie’s disease.
- Healthcare sites must be certified with the program and ensure that XIAFLEX is only dispensed for use by certified prescribers.
Further information is available at www.XIAFLEXREMS.com or 1-877-313-1235.
5.4 Hypersensitivity Reactions, Including Anaphylaxis
In the controlled portions of the clinical trials in Dupuytren’s contracture (Studies 1 and 2), a greater proportion of XIAFLEX-treated patients (15%) compared to placebo-treated patients (1%) had mild allergic reactions (pruritus) after up to 3 injections. The incidence of XIAFLEX-associated pruritus increased after more XIAFLEX injections in patients with Dupuytren’s contracture.
In the double-blind, placebo-controlled portions of the clinical trials in Peyronie’s disease (Studies 1 and 2), a greater proportion of XIAFLEX-treated patients (4%) compared to placebo-treated patients (1%) had localized pruritus after up to 4 treatment cycles (involving up to 8 XIAFLEX injection procedures). The incidence of XIAFLEX-associated pruritus was similar after each injection regardless of the number of injections administered.
Because XIAFLEX contains foreign proteins, severe allergic reactions to XIAFLEX can occur. Anaphylaxis was reported in a post-marketing clinical trial (Study 3) in one patient who had previous exposure to XIAFLEX for the treatment of Dupuytren’s contracture. Some patients with Dupuytren’s contracture developed IgE-anti-drug antibodies in greater proportions and higher titers with successive XIAFLEX injections. Healthcare providers should be prepared to address severe allergic reactions following XIAFLEX injections.
5.5 Risk of Bleeding in Patients with Abnormal Coagulation
In the XIAFLEX trials in Dupuytren’s contracture (Studies 1 and 2), 70% and 38% of XIAFLEX-treated patients developed an ecchymosis/contusion or an injection site hemorrhage, respectively (see Table 3). In the XIAFLEX controlled trials in Peyronie’s disease (Studies 1 and 2), 65.5% of XIAFLEX-treated patients developed penile hematoma, and 14.5% developed penile ecchymosis (see Table 4). Patients with abnormal coagulation (except for patients taking low-dose aspirin, e.g., up to 150 mg per day) were excluded from participating in these studies.
Therefore, the efficacy and safety of XIAFLEX in patients receiving anticoagulant medications (other than low-dose aspirin, e.g., up to 150 mg per day) within 7 days prior to XIAFLEX administration is not known. In addition, it is recommended to avoid use of XIAFLEX in patients with coagulation disorders, including patients receiving concomitant anticoagulants (except for low-dose aspirin).
-
6 ADVERSE REACTIONS
The following serious adverse reactions in patients with Dupuytren’s contracture are discussed in greater detail elsewhere in the labeling:
- Tendon ruptures or other serious injury to the injected extremity [see Warnings and Precautions (5.1)]
The following serious adverse reactions in patients with Peyronie’s disease are discussed in greater detail elsewhere in the labeling:
- Corporal rupture (penile fracture) and severe penile hematoma [see Warnings and Precautions (5.2)]
- In other XIAFLEX-treated patients, a combination of penile ecchymoses or hematoma, sudden penile detumescence, and/or a penile “popping” sound or sensation was reported, and in these cases, a diagnosis of corporal rupture cannot be excluded [see Warnings and Precautions (5.2)]
6.1 Clinical Studies Experience in Patients with Dupuytren’s Contracture
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Out of 1082 patients who received 0.58 mg of XIAFLEX in the controlled and uncontrolled portions of the XIAFLEX studies (2630 XIAFLEX injections), 3 (0.3%) patients had a flexor tendon rupture of the treated finger within 7 days of the injection.
The data described below are based on two pooled randomized, double-blind, placebo-controlled trials through Day 90 in patients with Dupuytren’s contracture (Studies 1 and 2). In these trials, patients were treated with up to 3 injections of 0.58 mg of XIAFLEX or placebo with approximately 4-week intervals between injections and the patients had finger extension procedures the day after injection, if needed, to facilitate disruption of the cord [see Clinical Studies (14)]. These trials were comprised of 374 patients of whom 249 and 125 received 0.58 mg of XIAFLEX and placebo, respectively. The mean age was 63 years, 80% were male and 20% were female, and 100% were white.
In the placebo-controlled portions of Studies 1 and 2 through Day 90, 98% and 51% of XIAFLEX-treated and placebo-treated patients had an adverse reaction after up to 3 injections, respectively. Over 95% of XIAFLEX-treated patients had an adverse reaction of the injected extremity after up to 3 injections. Approximately 81% of these local reactions resolved without intervention within 4 weeks of XIAFLEX injections. The adverse reaction profile was similar for each injection, regardless of the number of injections administered. However, the incidence of pruritus increased with more injections [see Warnings and Precautions (5.4)].
The most frequently reported adverse drug reactions (≥ 25%) in the XIAFLEX clinical trials in patients with Dupuytren’s contracture included edema peripheral (mostly swelling of the injected hand), contusion, injection site hemorrhage, injection site reaction, and pain in the treated extremity. Table 3 shows the incidence of adverse reactions that were reported in greater than or equal to 5% of XIAFLEX-treated patients and at a frequency greater than placebo-treated patients after up to 3 injections in the pooled placebo-controlled trials through Day 90 (Studies 1 and 2).
Table 3. Adverse Reactions Occurring in ≥ 5% of XIAFLEX-Treated Patients with Dupuytren’s Contracture and at a Greater Incidence than Placebo in the Placebo-Controlled Trials Through Day 90 After Up to 3 Injections a Most of these events were swelling of the injected hand. b Includes the terms: contusion (any body system) and ecchymosis c Includes the terms: injection site reaction, injection site erythema, injection site inflammation, injection site irritation, injection site pain, and injection site warmth d Includes the terms: injection site swelling and injection site edema e Includes the terms: pruritus and injection site pruritus f Includes the terms: lymphadenopathy and axillary mass Adverse Reaction XIAFLEX
N=249Placebo
N=125All Adverse Reactions 98% 51% Edema Peripherala 73% 5% Contusionb 70% 3% Injection Site Hemorrhage 38% 3% Injection Site Reactionc 35% 6% Pain in Extremity 35% 4% Tenderness 24% 0% Injection Site Swellingd 24% 6% Prurituse 15% 1% Lymphadenopathyf 13% 0% Skin Laceration 9% 0% Lymph Node Pain 8% 0% Erythema 6% 0% Axillary Pain 6% 0% Some patients developed vasovagal syncope after finger extension procedures.
The safety of two concurrent injections of XIAFLEX 0.58 mg into Dupuytren’s cords in the same hand was evaluated in a historically-controlled, open-label multi-center trial in 715 adult subjects with Dupuytren’s contracture (Study 3). In Study 3, finger extension procedures were performed approximately 24 to 72 hours after injection. The patient demographics were similar to Studies 1 and 2.
Out of 715 patients who received two concurrent injections of XIAFLEX 0.58 mg in the same hand (1450 XIAFLEX injections) in Study 3, one (0.1%) patient experienced a tendon rupture of the treated finger within 3 days of the injection.
Table 4 shows the incidence of adverse reactions that were reported in greater than or equal to 5% of XIAFLEX-treated patients after two concurrent injections of XIAFLEX in the same hand through Day 60 in Study 3.
Table 4. Adverse Reactions Occurring in ≥5.0% of Subjects Who Received Two Concurrent Injections of XIAFLEX in Study 3 Adverse Reaction XIAFLEX
N=715Subjects with ≥1 adverse reaction 95% Edema peripheral 77% Contusion 59% Pain in extremity 51% Laceration 22% Pruritus 15% Injection site pain 14% Lymphadenopathy 13% Blood blister 12% Injection site hematoma 8% Axillary pain 7% Injection site hemorrhage 6% Injection site swelling 5% Ecchymosis 5% Safety of Retreatment of Recurrent Contractures
An observational, open label study was conducted in subjects who had participated in XIAFLEX clinical trials for Dupuytren’s contracture (Study 4). A subset of patients who had recurrence of contracture in a joint that was previously successfully treated with XIAFLEX in Study 4 were retreated (Study 5). No new safety signals were identified among subjects who were retreated with XIAFLEX.
6.2 Clinical Studies Experience in Patients with Peyronie’s Disease
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In the controlled and uncontrolled clinical studies of XIAFLEX in Peyronie’s disease, 1044 patients received a total of 7466 XIAFLEX injections.
Corporal Rupture and Other Serious Penile Injury
- Corporal rupture was reported as an adverse reaction after XIAFLEX injections in 5 of 1044 (0.5%) XIAFLEX treated patients.
- In other XIAFLEX-treated patients (9 of 1044; 0.9%), a combination of penile ecchymoses or hematoma, sudden penile detumescence, and/or a penile “popping” sound or sensation was reported, and in these cases, a diagnosis of corporal rupture cannot be excluded. These patients were managed without surgical intervention, but the long-term consequences are unknown.
- Severe penile hematoma was also reported as an adverse reaction in 39 of 1044 patients (3.7%) in the controlled and uncontrolled clinical trials in Peyronie’s disease [see Adverse Reactions (6)].
The data described below are based on two identical, pooled, randomized, double-blind, placebo-controlled, multi-center trials through Day 365 in patients with Peyronie’s disease (Studies 1 and 2). These trials included 832 patients of whom 551 and 281 received XIAFLEX and placebo, respectively. In these trials, patients were given up to 4 treatment cycles of XIAFLEX or placebo. In each cycle, two injections of XIAFLEX or two injections of placebo were administered 1 to 3 days apart. A penile modeling procedure was performed at the study site on patients 1 to 3 days after the second injection of the cycle. The treatment cycle was repeated at approximately 6-week intervals up to three additional times, for a maximum of 8 total injection procedures and 4 total modeling procedures [see Clinical Studies (14.2)].
The majority of Peyronie’s patients experienced at least one adverse reaction (92% XIAFLEX-treated patients, 61% placebo-treated). Most adverse reactions were local events of the penis and groin and the majority of these events were of mild or moderate severity, and most (79%) resolved within 14 days of the injection. The adverse reaction profile was similar after each injection, regardless of the number of injections administered.
The most frequently reported adverse drug reactions (≥ 25%) in the XIAFLEX clinical trials in patients with Peyronie’s disease were penile hematoma, penile swelling, and penile pain. Table 5 shows the incidence of adverse reactions that were reported in greater than or equal to 1% of XIAFLEX-treated patients and at a frequency greater than placebo-treated patients after up to 8 injections in the pooled placebo-controlled trials through Day 365.
Table 5. Adverse Reactions Occurring in ≥ 1% of XIAFLEX-Treated Patients with Peyronie’s disease and at a Greater Incidence than Placebo After Up to Four Treatment Cycles in Studies 1 and 2 Combined a Includes: injection site hematoma and penile hematoma were reported with the verbatim term of penile bruising or injection site bruising in 87% of subjects. b Includes: injection site swelling, penile edema, penile swelling, local swelling, scrotal swelling, and injection site edema. c Includes: injection site pain, penile pain, and injection site discomfort. d Includes: contusion, ecchymoses, penile hemorrhage, and injection site hemorrhage. Adverse Reaction XIAFLEX
N=551Placebo
N=281All Adverse Reactions 84.2% 36.3% Penile hematomaa 65.5% 19.2% Penile swellingb 55.0% 3.2% Penile painc 45.4% 9.3% Penile ecchymosesd 14.5% 6.8% Blood blister 4.5% 0 Penile blister 3.3% 0 Pruritus genital 3.1% 0 Painful erection 2.9% 0 Erectile dysfunction 1.8% 0.4% Skin discoloration 1.8% 0 Procedural pain 1.6% 0.7% Injection site vesicles 1.3% 0 Localized edema 1.3% 0 Dyspareunia 1.1% 0 Injection site pruritus 1.1% 0 Nodule 1.1% 0 Suprapubic pain 1.1% 0 Severe penile hematoma or severe injection site hematoma were reported in 33/551 (6.0%) of XIAFLEX-treated patients and 0/281 (0%) of placebo-treated patients, in Studies 1 and 2 combined.
Reports of penile “popping” sounds or sensations
A popping noise or popping sensation in the penis, sometimes described as “snapping” or “cracking”, and sometimes accompanied by detumescence, hematoma and/or pain, were reported in 73/551 (13.2%) XIAFLEX-treated patients and 1/281 (0.3%) placebo-treated patients.There were no clinically meaningful differences in the incidence of adverse events following treatment with XIAFLEX based on the severity of baseline erectile dysfunction or concomitant phosphodiesterase type 5 (PDE5) inhibitor use.
XIAFLEX was not associated with shortening of penile length in clinical trials in the treatment of Peyronie’s disease.
6.3 Immunogenicity
During clinical studies in Dupuytren’s contracture and Peyronie’s disease, patients were tested at multiple time points for antibodies to the protein components of XIAFLEX (AUX-I and AUX-II).
In the Dupuytren’s contracture clinical studies (Studies 1 and 2), at 30 days post the first injection of XIAFLEX 0.58 mg, 92% of patients had antibodies against AUX-I detected and 86% of patients had antibodies against AUX-II detected. After the fourth injection of XIAFLEX, every XIAFLEX-treated patient developed high titers of antibodies to both AUX-I and AUX-II. After five years more than 90 percent of patients remained seropositive for anti-AUX-I and anti-AUX-II antibody (Study 4). Neutralizing antibodies were assayed for all patients (204) in Study 1. Neutralizing antibodies to AUX-I or AUX-II, were detected in 10% and 21%, respectively, of patients treated with XIAFLEX. Among patients in Study 3 who reported no prior exposure to XIAFLEX, 97% of patients had antibodies against AUX-I and AUX-II after two concurrent doses of XIAFLEX 0.58 mg (total dose of 1.16 mg) in the same hand. In Study 5, treatment of recurrent contractures with XIAFLEX resulted in similar immunogenicity results as seen in Studies 1 and 2.
In the Peyronie’s disease clinical studies, at 6 weeks after the first treatment cycle of XIAFLEX 0.58 mg, approximately 75% of patients had antibodies against AUX-I and approximately 55% of patients had antibodies against AUX-II. Six weeks after the eighth injection (fourth treatment cycle) of XIAFLEX, >99% of XIAFLEX-treated patients developed high titers of antibodies to both AUX-I and AUX-II. Neutralizing antibodies were assayed for a subset of 70 samples selected to be representative of high and low titer binding antibody responses at week 12 of treatment. For each subject in whom a Week 12 sample was selected, the corresponding Week 6, 18, 24, and 52 samples were assayed if they were also binding antibody positive. Neutralizing antibodies to AUX-I or AUX-II, were detected in 60% and 51.8%, respectively, of patients tested.
In patients treated for these two indications, there was no apparent correlation of antibody frequency, antibody titers, or neutralizing status to clinical response or adverse reactions.
Since the protein components in XIAFLEX (AUX-I and AUX-II) have some sequence homology with human matrix metalloproteinases (MMPs), anti-product antibodies could theoretically interfere with human MMPs. In vitro studies showed no evidence of cross-reactivity between anti-drug-antibody positive patient sera and a series of relevant MMPs. In addition, no clinical safety concerns related to the inhibition of endogenous MMPs have been observed.
Immunogenicity assay results are highly dependent on the sensitivity and specificity of the assay used in detection and may be influenced by several factors, including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to collagenase clostridium histolyticum with the incidence of antibodies to other products may be misleading.
-
7 DRUG INTERACTIONS
Anticoagulant drugs: XIAFLEX should be used with caution in patients receiving concomitant anticoagulants (except for low-dose aspirin) [see Warnings and Precautions (5.5)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
There are no adequate and well-controlled studies of XIAFLEX in pregnant women. Because animal reproduction studies are not always predictive of human response, XIAFLEX should be used during pregnancy only if clearly needed.Risk Summary
Based on animal data, XIAFLEX is not predicted to increase the risk for major developmental abnormalities in humans.Human Data
Human pharmacokinetic studies showed that XIAFLEX levels were not quantifiable in the systemic circulation following injection into a Dupuytren’s cord.Low levels of XIAFLEX were quantifiable in the plasma of evaluable male subjects for up to 30 minutes following administration of XIAFLEX into the penile plaque of subjects with Peyronie’s disease [see Clinical Pharmacology (12.3)].
Almost all patients develop anti-product antibodies (anti-AUX-I and anti-AUX-II) after treatment with XIAFLEX, and the clinical significance of anti-product antibody formation on a developing fetus is not known [see Adverse Reactions (6)].
Animal Data
Reproduction studies have been performed in rats with intravenous exposures up to approximately 11 times the maximum recommended human dose (MRHD) of XIAFLEX on a mg/m2 basis, and have revealed no evidence of impaired fertility or harm to the fetus due to collagenase clostridium histolyticum.8.3 Nursing Mothers
It is not known whether collagenase clostridium histolyticum is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when XIAFLEX is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of XIAFLEX in pediatric patients less than 18 years old have not been established.
8.5 Geriatric Use
Of the 249 XIAFLEX-treated patients in the double-blind, placebo-controlled, clinical trials in Dupuytren’s contracture (Studies 1 and 2), 104 (42%) were 65 years of age or older and 9% were 75 years of age or older. Of the 551 XIAFLEX-treated patients in the double-blind, placebo-controlled, clinical trials in Peyronie’s disease (Studies 1 and 2), 100 (18%) were 65 years of age or older and 5 (0.9 %) were 75 years of age or older. No overall differences in safety or effectiveness of XIAFLEX were observed between these patients and younger patients.
-
10 OVERDOSAGE
The effects of overdose of XIAFLEX are unknown. It is possible that multiple simultaneous or excessive doses of XIAFLEX may cause more severe local effects than the recommended doses including serious adverse reactions in the injected area (e.g., tendon ruptures or corporal ruptures dependent on the injection site). Supportive care and symptomatic treatment are recommended in these circumstances.
-
11 DESCRIPTION
XIAFLEX contains purified collagenase clostridium histolyticum, consisting of two microbial collagenases in a defined mass ratio, Collagenase AUX-I and Collagenase AUX-II, which are isolated and purified from the fermentation of Clostridium histolyticum bacteria.
Collagenase AUX-I is a single polypeptide chain consisting of approximately 1000 amino acids of known sequence. It has an observed molecular weight of 114 kiloDaltons (kDa). It belongs to the class I Clostridium histolyticum collagenases.
Collagenase AUX-II is a single polypeptide chain consisting of approximately 1000 amino acids of deduced sequence. It has an observed molecular weight of 113 kDa. It belongs to the class II Clostridium histolyticum collagenases.
XIAFLEX is supplied as a sterile lyophilized powder (white cake) intended for reconstitution with the supplied sterile diluent (0.3 mg/mL calcium chloride dihydrate in 0.9% sodium chloride) prior to intralesional injection into a Dupuytren’s cord or a Peyronie’s plaque.
XIAFLEX is available in single-use, glass vials containing 0.9 mg of collagenase clostridium histolyticum. Each vial also contains 0.5 mg of hydrochloric acid, 18.5 mg of sucrose, and 1.1 mg of tromethamine.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Collagenases are proteinases that hydrolyze collagen in its native triple helical conformation under physiological conditions, resulting in lysis of collagen deposits.
Injection of XIAFLEX into a Dupuytren’s cord, which is comprised mostly of collagen, may result in enzymatic disruption of the cord.
The signs and symptoms of Peyronie’s disease are caused by a collagen plaque. Injection of XIAFLEX into a Peyronie’s plaque, which is comprised mostly of collagen, may result in enzymatic disruption of the plaque. Following this disruption of the plaque, penile curvature deformity and patient bother caused by Peyronie’s disease are reduced [see Clinical Studies (14.2)].
Results of in vitro studies, including those of explant tissues containing Peyronie’s plaques, suggest that XIAFLEX disrupts the predominant collagen found in plaques (Types I and III). At higher doses and longer incubation times, non-fibrillar Type IV collagen was affected causing collagen lysis in small veins, but did not cause structural damage to arteries, nerves or large veins which contain Type IV collagen in in vitro or in vivo studies.
Results of in vitro studies suggest that the collagenases (AUX-I and AUX-II) worked synergistically to provide hydrolyzing activity towards collagen. However, there are no clinical data regarding the relative contributions of the individual collagenases (AUX-I or AUX-II) to the efficacy of XIAFLEX in the treatment of Dupuytren’s contracture or Peyronie’s disease.
Collagen fragments generated from clostridial collagenase have been shown to generate increased vascular permeability, inflammatory responses, and regenerative changes. However, the effects of the formation of the collagen fragments derived from the collagen plaque are unknown.
12.3 Pharmacokinetics
Following administration of either a single injection of XIAFLEX 0.58 mg into a Dupuytren’s cord in 20 patients or two concurrent injections of XIAFLEX 0.58 mg into Dupuytren’s cords of 12 patients, no quantifiable levels of XIAFLEX (AUX-I or AUX-II) were detected in plasma up to 30 days post injection.
Following each of two intralesional administrations, separated by 24 hours, of XIAFLEX 0.58 mg into the penile plaque of 19 subjects with Peyronie’s disease, plasma levels of AUX-I and AUX-II in subjects with quantifiable levels (79% and 40% for AUX-I and AUX-II, respectively) were minimal and short-lived. The maximal plasma concentrations of AUX-I and AUX-II were <29 ng/mL and <71 ng/mL, respectively, and were observed approximately within 10 minutes after injection. All plasma levels were below the limits of quantification within 30 minutes following dosing. There was no evidence of accumulation following two sequential injections of XIAFLEX administered 24 hours apart. No subject had quantifiable plasma levels 15 minutes after modeling of plaque on Day 3 (i.e., 24 hours after Injection 2 on Day 2).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of collagenase clostridium histolyticum have not been conducted.Mutagenesis
Purified collagenase clostridium histolyticum was not mutagenic in Salmonella typhimurium (AMES test) and was not clastogenic in both an in vivo mouse micronucleus assay and an in vitro chromosomal aberration assay in human lymphocytes.Impairment of Fertility
Collagenase clostridium histolyticum did not impair fertility and early embryonic development when administered intravenously in rats at exposures up to approximately 11 times the maximum recommended human dose (MRHD) on a mg/m2 basis.13.2 Animal Toxicology and/or Pharmacology
Single or repeat-dose intravenous studies of collagenase clostridium histolyticum in rats were conducted to evaluate the toxicological impact of injection of collagenase clostridium histolyticum directly into the systemic circulation. Dose-dependent liver toxicity was noted at exposures greater than or equal to approximately 11 times the MRHD on a mg/m2 basis as characterized by elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, increased liver weights, mild regenerative anemia with secondary changes in the spleen and histologic findings of chronic active inflammation, hemorrhage/hematoma, fibrosis, bile duct hyperplasia and/or hepatocellular necrosis. The histologic findings remained unresolved following a 2-week recovery period, while the other findings resolved completely. Animals that were found dead or prematurely euthanized had histopathological findings of hemorrhage and necrosis in the liver and extramedullary hematopoiesis in the liver and/or spleen. Death occurred at approximately 25 times the MRHD on a mg/m2 basis between study days 7 and 15 following 8 repeat intravenous administrations of collagenase clostridium histolyticum over 16 days or after single intravenous doses at approximately 40 times the MRHD on a mg/m2 basis.
In intermittent 13-week subcutaneous repeat-dose studies in rats or dogs administered doses up to approximately 3 times the MRHD on a mg/m2 basis, respectively, there was no evidence of systemic toxicity. In a single-dose phase or 61-day repeat-dose phase (3 times a week every 3 weeks for 3 cycles) study of intrapenile administration of collagenase clostridium histolyticum in dogs at exposures lower than or equal to the MRHD on a mg/m2 basis, there was no evidence of systemic toxicity.
-
14 CLINICAL STUDIES
14.1 Dupuytren’s Contracture
The efficacy of 0.58 mg of XIAFLEX was evaluated in two randomized, double-blind, placebo-controlled, multi-centered trials in 374 adult patients with Dupuytren’s contracture (Studies 1 and 2). At study entry, patients must have had: (1) a finger flexion contracture with a palpable cord of at least one finger (other than the thumb) of 20° to 100° in a metacarpophalangeal (MP) joint or 20° to 80° in a proximal interphalangeal (PIP) joint and (2) a positive “table top test” defined as the inability to simultaneously place the affected finger(s) and palm flat against a table top. Patients could not have received a surgical treatment (e.g., fasciectomy, fasciotomy) on the selected primary joint within 90 days before the first injection of study medication and patients could not have received anticoagulation medication (except for up to 150 mg of aspirin per day) within 7 days before the first injection of study medication.
The cord affecting the selected primary joint received up to 3 injections of 0.58 mg of XIAFLEX or placebo on Days 0, 30, and 60. About 24 hours after each injection of study medication, if needed, the investigator manipulated (extended) the treated finger in an attempt to facilitate rupture of the cord (finger extension procedure). Following manipulation, patients were fitted with a splint, instructed to wear the splint at bedtime for up to 4 months, and instructed to perform a series of finger flexion and extension exercises each day.
Table 6 shows the baseline disease characteristics of patients with Dupuytren’s contracture in Studies 1 and 2.
Table 6. Baseline Disease Characteristics of Patients with Dupuytren’s Contracture 1 Prior surgery for Dupuytren’s contracture included fasciotomy and fasciectomy Study 1 Study 2 Proportion of patients with prior surgery for Dupuytren’s contracture1 38% 53% Proportion of patients with prior surgery for Dupuytren’s contracture on the same finger as the primary joint1 8% 18% Mean number of affected joints 3.0 3.3 In Studies 1 and 2, the primary endpoint was to evaluate the proportion of patients who achieved a reduction in contracture of the selected primary joint (MP or PIP) to within 0º to 5º of normal, 30 days after the last injection of that joint on Days 30, 60, or 90 (after up to 3 injections). As shown in Table 7, a greater proportion of XIAFLEX-treated patients compared to placebo-treated patients achieved the primary endpoint.
Table 7. Percentage of Patients Who Achieved Reduction in Contracture of the Primary Joint to 0° to 5° After Up to 3 Injections in Studies 1 and 2a a Patients may have received up to 3 injections of study medication into the cords associated with contracture of the primary joints on Days 0, 30, and 60. Assessments were made 30 days after the last injection (on Days 30, 60, or 90). b For XIAFLEX-treated patients, the mean (±SD) number of injections given to the cord associated with the contracture was 1.7 (±0.8) in the 90-day controlled period in each trial. c MP joints are metacarpophalangeal joints d PIP joints are proximal interphalangeal joints e 95% confidence interval Treated Joint Study 1 Study 2 XIAFLEXb Placebo XIAFLEXb Placebo All Joints (MP and PIP)c,d
Difference (CIe)N=203 N=103 N=45 N=21 64%
57% (47%, 67%)7%
-44%
40% (14%, 62%)5%
-MP Jointsc
Difference (CIe)N=133 N=69 N=20 N=11 77%
69% (57%, 79%)7%
-65%
56% (19%, 83%)9%
-PIP Jointsd
Difference (CIe)N=70 N=34 N=25 N=10 40%
34% (14%, 52%)6%
-28%
28% (-10%, 61%)0%
-The proportion of patients who achieved a contracture reduction of the primary joint to 0º to 5º after the first injection was 39% and 1% in Study 1 and 27% and 5% in Study 2 in the XIAFLEX and placebo groups respectively.
XIAFLEX-treated patients, compared to placebo-treated patients, showed a greater increase from baseline in the range of motion of MP and PIP joints (see Table 8).
Table 8. Mean Increase in Range of Motion from Baseline in Degrees After Up to 3 Injections in Studies 1 and 2a aPatients may have received up to 3 injections of study medication into the cords associated with contracture of the primary joints on Days 0, 30, and 60. Assessments were made 30 days after the last injection (on Days 30, 60, or 90). Baseline and final range of motion degree values are expressed in mean (SD). bMP = Metacarpophalangeal joint cPIP = Proximal interphalangeal joint Range of Motion = Degrees of Full Flexion minus Degrees of Fixed Extension Not all patients had range of motion values at both time points. Treated Joint Study 1 Study 2 XIAFLEX Placebo XIAFLEX Placebo All Joints b,c N=196 N=102 N=45 N=21 Baseline 44 (20) 45 (19) 40 (15) 44 (16) Final 80 (20) 50 (22) 76 (18) 52 (20) Increase 36 (21) 4 (15) 35 (18) 8 (15) MP Joints b N=129 N=68 N=20 N=11 Baseline 43 (20) 46 (19) 40 (12) 41 (21) Final 83 (16) 50 (21) 80 (11) 50 (22) Increase 41 (20) 4 (13) 40 (13) 9 (15) PIP Joints c N=67 N=34 N=25 N=10 Baseline 46 (20) 44 (18) 41 (18) 47 (10) Final 75 (24) 49 (24) 73 (21) 54 (18) Increase 28 (22) 5 (19) 32 (20) 7 (16) Recurrence
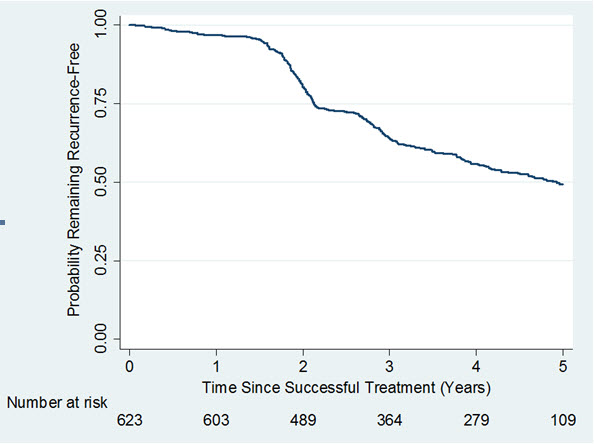
A long term, observational, Year 2 to Year 5 follow-up study (Study 4) was undertaken to evaluate recurrence of contracture and long-term safety in subjects who received up to 8 single injections of XIAFLEX 0.58mg in a previous Phase 3 open-label or double-blind with open-label extension study. Of the 950 patients eligible for Study 4, only 645 patients enrolled. Of the 645 patients enrolled, 30% discontinued the study. Recurrence was assessed in successfully treated joints (i.e., subjects had a reduction in contracture to 5º or less at the Day 30 evaluation after the last injection of XIAFLEX in a previous study) and was defined as an increase in joint contracture by at least 20º in the presence of a palpable cord, or the joint underwent medical or surgical intervention primarily to correct a new or worsening Dupuytren’s contracture in that joint. Data on remaining recurrence free following successful treatment with XIAFLEX are provided in Figure 1.
Figure 1. Kaplan-Meier Plot Displaying Estimated Probability of Remaining Recurrence-Free over Time in the Observational Study 4 among Joints that were Successfully Treated in a Previous Study
Retreatment of Recurrent Contractures
Study 5 retreated a subset of patients from Study 4 for a joint that was previously successfully treated but had recurrence. Patients in Study 5 received up to 3 injections of XIAFLEX (0.58 mg). Of the 91patients eligible for Study 5, 52 patients enrolled. In Study 5, 65% of recurrent MP joints and 45% of recurrent PIP joints achieved clinical success after retreatment with up to three injections of XIAFLEX. There was no control group for comparison in Study 5.
14.2 Peyronie’s Disease
The efficacy of XIAFLEX was evaluated in two randomized, double-blind, placebo-controlled, multi-centered trials in 832 adult males with Peyronie’s disease (Studies 1 and 2). At study entry, patients must have had penile curvature deformity of at least 30 degrees in the stable phase of Peyronie’s disease. Patients were excluded if they had a ventral curvature deformity, an isolated hourglass deformity or a calcified plaque that could have interfered with the injection technique. At baseline, penile pain was either not present or was mild in most (98%) patients.
In these trials, patients were given up to 4 treatment cycles of XIAFLEX or placebo (weeks 0, 6, 12, 18), and were followed in a non-treatment follow-up period (weeks 24 -52). In each treatment cycle, two injections of XIAFLEX or two injections of placebo were administered 1 to 3 days apart. A penile modeling procedure was performed on patients at the study site 1 to 3 days after the second injection of the cycle. The treatment cycle was repeated at approximately six-week intervals for up to three additional times, for a maximum of 8 total injection procedures and 4 total modeling procedures. In addition, patients were instructed to perform penile modeling at home for six weeks after each treatment cycle [see Medication Guide].
Table 9 shows the baseline disease characteristics of patients with Peyronie’s disease in Studies 1 and 2.
Table 9. Baseline Disease Characteristics of Patientsa with Peyronie’s Disease (PD) a Subjects were from ITT population and received at least one dose of study drug in Study 1 or 2 b Each PDQ assessment required subjects to have had vaginal intercourse in the 3 months prior to completion c Higher scores represent worse symptoms Study 1 Study 2 XIAFLEX
N=277Placebo
N=140XIAFLEX
N=274Placebo
N=141Mean age (years) (Min-Max) 57.9
(28 - 79)58.2
(30 - 81)57.3
(23 - 84)57.6
(33 - 78)Mean duration of PD (years) (Min-Max) 3.9
(1.0 - 35.9)4.8
(1.0 - 50.8)4.2
(1.1 - 30.9)3.4
(1.1 - 47.1)Mean Penile Curvature Deformity (degrees) (Min-Max) 48.8
(30-90)49.0
(30-89)51.3
(30-90)49.6
(30-85)Peyronie’s Disease Questionnaire (PDQ) b, – Mean Patient-Reported PD Bother Score (range: 0-16) c 7.5 7.4 7.4 8.4 History of Erectile Dysfunction N (%) 128 (46.2) 75 (53.6) 134 (48.9) 76 (53.9) Before the first dose of study drug was administered, eligible subjects were stratified by the degree of curvature deformity (30 to 60 degrees, and 61 to 90 degrees) and then randomized into two treatment groups to receive either XIAFLEX or placebo in a 2:1 ratio. The efficacy population (modified intent-to-treat (mITT) population) comprised a total of 612 intent-to-treat subjects who had both a curvature deformity measurement and a PDQ assessment at baseline, and at one or more subsequent time points in Studies 1 and 2, and had engaged in vaginal intercourse within 3 months prior to each PDQ assessment.
In Studies 1 and 2, the co-primary endpoints were:
- the percent change from baseline to Week 52 in penile curvature deformity and;
- the change from baseline to Week 52 in the Bother domain score of the PDQ
The Bother domain score is a composite of the following patient-reported items: concern about erection pain, erection appearance, and the impact of Peyronie’s disease on intercourse and on frequency of intercourse.
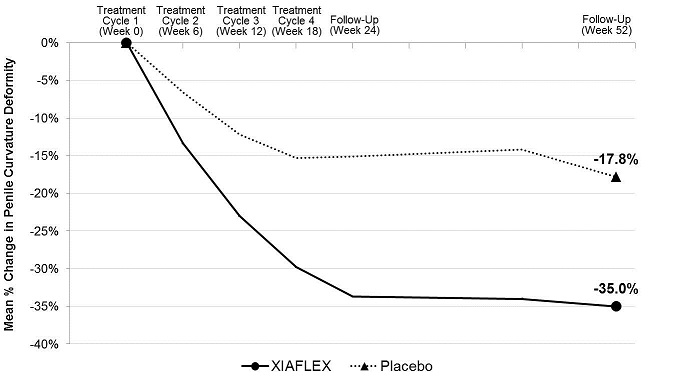
Penile curvature deformity (co-primary endpoint)
XIAFLEX treatment significantly improved penile curvature deformity in patients with Peyronie’s disease compared with placebo (see Table 10). The improvement in curvature deformity was numerically similar among subjects with baseline curvature deformity from 30 to 60 degrees and those with curvature deformity from 61 to 90 degrees.Table 10. Mean Percent Change in Penile Curvature Deformity from Baseline to Week 52 - Studies 1 and 2 a Mean percent change, treatment difference, 95% CI, and p-value were based on an ANOVA model with factors for treatment, stratum of baseline penile curvature, and their interaction and using last observation carried forward (LOCF) in the modified intent-to-treat (mITT) population. The mITT population was defined as all randomized subjects who had both a penile curvature deformity measurement and a PDQ assessment at baseline and at one or more subsequent time points. bp-value < 0.01 Study 1 Study 2 XIAFLEX
N=199Placebo
N=104XIAFLEX
N=202Placebo
N=107Baseline Mean (degrees) 48.8° 49.0° 51.3° 49.6° Mean Percent Change a -35.0% -17.8% -33.2% -21.8% Treatment Difference (95% CI) -17.2%b
(-26.7%, -7.6%)-11.4% b
(-19.5%, -3.3%)Figure 2. Mean Percent Change in Penile Curvature Deformity –Study 1
ANOVA model –adjusted values with factors for treatment, stratum of baseline penile curvature, and their interaction and using last observation carried forward (LOCF) in the modified intent-to-treat (mITT) population.
Figure 3. Mean Percent Change in Penile Curvature Deformity – Study 2
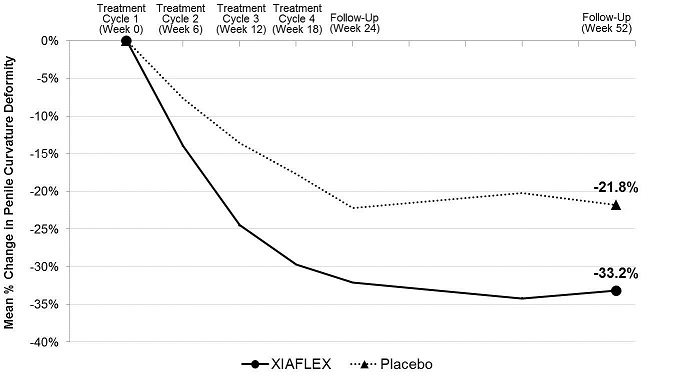
ANOVA model –adjusted values with factors for treatment, stratum of baseline penile curvature, and their interaction and using last observation carried forward (LOCF) in the modified intent-to-treat (mITT) population.
Peyronie’s Disease Questionnaire Bother domain score (co-primary endpoint)
XIAFLEX significantly reduced patient-reported bother associated with Peyronie’s disease compared with placebo (see Table 11). The reduction in the bother domain score was numerically similar between patient groups stratified by degree of baseline curvature deformity (30 to 60 degrees, and 61 to 90 degrees).Table 11. Mean Change in Peyronie’s Disease Bother Domain Score from Baseline to Week 52 – Studies 1 and 2 a Mean change, treatment difference, 95% CI, and p-value all based on an ANOVA model with factors for treatment, stratum of baseline penile curvature, and their interaction and using last observation carried forward (LOCF) in the modified intent-to-treat (mITT) population. The mITT population was defined as all randomized subjects who had both a penile curvature deformity measurement and a PDQ assessment at baseline and at one or more subsequent time points. bp-value < 0.05. Study 1 Study 2 XIAFLEX
N=199Placebo
N=104XIAFLEX
N=202Placebo
N=107Baseline Mean 7.5 7.4 7.4 8.2 Mean Change a -2.8 -1.6 -2.6 -1.5 Treatment Difference
(95% CI)-1.2b
(-2.4, -0.03)-1.1b
(-2.1, -0.002)Figure 4. Mean Change in Patient-Reported Peyronie’s Disease Bother Domain Score – Study 1
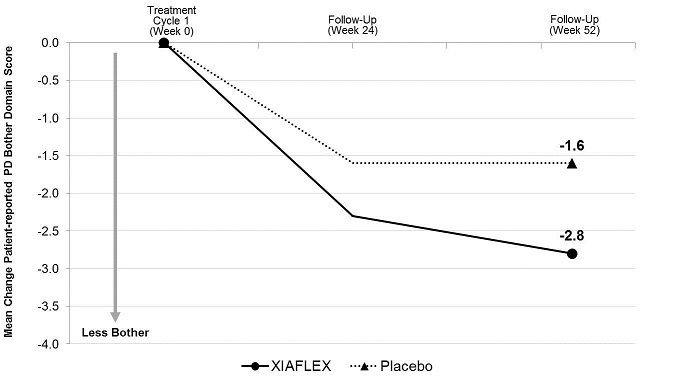
ANOVA model –adjusted values with factors for treatment, stratum of baseline penile curvature, and their interaction and using last observation carried forward (LOCF) in the modified intent-to-treat (mITT) population.
Figure 5. Mean Change in Patient-Reported Peyronie’s Disease Bother Domain Score – Study 2
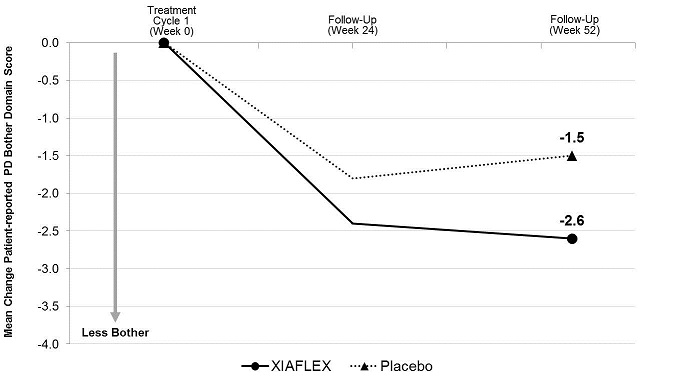
ANOVA model –adjusted values with factors for treatment, stratum of baseline penile curvature, and their interaction and using last observation carried forward (LOCF) in the modified intent-to-treat (mITT) population.
There were no clinically meaningful differences in the mean percent improvement in curvature deformity or mean reduction in the bother domain score following treatment with XIAFLEX based on the severity of baseline erectile dysfunction or concomitant phosphodiesterase type 5 (PDE5) inhibitor use.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
XIAFLEX is available in single-use, glass vials containing 0.9 mg of collagenase clostridium histolyticum as a sterile, lyophilized powder.
Sterile diluent for reconstitution is available in single-use, glass vials containing 3 mL of 0.3 mg/mL calcium chloride dihydrate in 0.9% sodium chloride.
NDC Number Package Size 66887-003-01 Single-use package:
1 carton containing a single-use vial of XIAFLEX and a single-use vial of sterile diluent66887-003-02 Dual-Pack (two single-use packages):
1 box containing 2 cartons, each containing a single-use vial of XIAFLEX and a single-use vial of sterile diluentStorage and Stability
Prior to reconstitution, the vials of XIAFLEX and diluent should be stored in a refrigerator at 2° to 8°C (36° to 46°F) [see Dosage and Administration (2.1, 2.2)]. Do not freeze.The reconstituted XIAFLEX solution can be kept at room temperature (20° to 25°C/68° to 77°F) for up to one hour or refrigerated at 2° to 8°C (36° to 46°F) for up to 4 hours prior to administration [see Dosage and Administration (2.1, 2.2)].
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
17.1 Patient Counseling for Dupuytren’s Contracture
Advise patients of the following:
- Serious complications of XIAFLEX injection include tendon rupture, serious ligament damage, or skin laceration that may result in the inability to fully bend the finger and may require surgery to correct the complication.
- XIAFLEX injection is likely to result in swelling, bruising, bleeding, and/or pain of the injected site and surrounding tissue.
After the XIAFLEX injections, instruct patients:
- Not to flex or extend the fingers of the injected hand to reduce extravasation of XIAFLEX out of the cord(s).
- Not to attempt to disrupt the injected cord(s) by self-manipulation.
- To elevate the injected hand until bedtime.
- To promptly contact their physician if there is evidence of infection (e.g., fever, chills, increasing redness or edema), sensory changes in the treated finger(s), trouble bending the finger(s) after the swelling goes down (symptoms of tendon rupture), or skin laceration.
- To return to their healthcare provider’s office one to three days after the injection visit for an examination of the injected hand and for possible finger extension procedure(s) to disrupt the cord.
Following the finger extension procedure(s) and fitting patient with a splint, instruct patients:
- Not to perform strenuous activity with the injected hand until advised to do so.
- To wear the splint at bedtime for up to 4 months.
- To perform a series of finger flexion and extension exercises each day.
17.2 Patient Counseling for Peyronie’s Disease
Advise patients of the following:
- Serious complications of XIAFLEX injection include corporal rupture and penile hematoma and may require surgery to correct the complication.
After the XIAFLEX injections, instruct the patient:
- That their penis may appear bruised and/or swollen
- That they may have mild-to-moderate penile pain that can be relieved by taking over-the-counter pain medications
- To promptly contact their physician if, at any time, they have severe pain or severe swelling of the penis, severe purple bruising and swelling of the penis, difficulty urinating or blood in the urine, or sudden loss of the ability to maintain an erection. These symptoms may be accompanied by a popping or cracking sound from the penis
- To return to their healthcare provider’s office when directed for further injection(s) and/or penile modeling procedure(s)
- To not have sex between the first and second injections of a treatment cycle
- To wait four weeks after the second injection of a treatment cycle before resuming sexual activity, provided pain and swelling have subsided
- To perform gentle, at home modeling activities, as recommended by their physician
- To refrain from using a vacuum erection device during treatment with Xiaflex
- To avoid abdominal straining associated with situations, such as straining during bowel movements
Provide the patient instructions on the appropriate technique to perform penile modeling activities at home, as described in “What You Need to Know About XIAFLEX Treatment for Peyronie's Disease: A Patient Guide”, and give the patient a copy.
Distributed by:
Endo Pharmaceuticals Inc.
Malvern, PA 19355Manufactured by:
Auxilium Pharmaceuticals, LLC
Malvern, PA 19355US License No. 1816
US Patent Nos. 7,811,560; RE39,941; and 6,022,539
Revised: 11/2019
-
MEDICATION GUIDE
Medication Guide
XIAFLEX® (Zī a flex)
(collagenase clostridium histolyticum)
For injection, for intralesional use
XIAFLEX is approved for two uses: Dupuytren's contracture and Peyronie's disease. Information is provided separately for each use. Use for treating Dupuytren's contracture is described first, followed by use for treating Peyronie's disease.
Read this Medication Guide before you receive XIAFLEX for the treatment of Dupuytren’s contracture and each time you get an injection. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about XIAFLEX for the treatment of Dupuytren’s contracture?
XIAFLEX can cause serious side effects, including:
- Tendon rupture or ligament damage. Receiving an injection of XIAFLEX may cause damage to a tendon or ligament in your hand and cause it to break or weaken. This could require surgery to fix the damaged tendon or ligament. Call your healthcare provider right away if you have trouble bending your injected finger (towards the wrist) after the swelling goes down or you have problems using your treated hand after your follow-up visit.
- Nerve injury or other serious injury of the hand. Call your healthcare provider right away if you get numbness, tingling, increased pain, or tears in the skin (laceration) in your treated finger or hand after your injection or after your follow-up visit.
- Hypersensitivity reactions, including anaphylaxis. Severe allergic reactions can happen in people who receive XIAFLEX, because it contains foreign proteins.
Call your healthcare provider right away if you have any of these symptoms of an allergic reaction after an injection of XIAFLEX:
- hives
- swollen face
- breathing trouble
- chest pain
- low blood pressure
- dizziness or fainting
What is XIAFLEX?
XIAFLEX is a prescription medicine used to treat adults with Dupuytren’s contracture when a “cord” can be felt.
It is not known if XIAFLEX is safe and effective in children under the age of 18.
Who should not receive XIAFLEX?
Do not receive XIAFLEX if you:
- are allergic to collagenase clostridium histolyticum, or any of the ingredients in XIAFLEX, or to any other collagenase product. See the end of this Medication Guide for a complete list of ingredients in XIAFLEX.
Talk to your healthcare provider before receiving this medicine if you have any of these conditions.
What should I tell my healthcare provider before receiving XIAFLEX?
Before receiving XIAFLEX, tell your healthcare provider if you:
- have had an allergic reaction to a XIAFLEX injection in the past
- have a bleeding problem
- have received XIAFLEX to treat another condition
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if XIAFLEX will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if XIAFLEX passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive XIAFLEX.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Using XIAFLEX with certain other medicines can cause serious side effects.
Especially tell your healthcare provider if you take:
- medicines to thin your blood (anticoagulants). If you are told to stop taking a blood thinner before your XIAFLEX injection, your healthcare provider should tell you when to restart the blood thinner.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How will I receive XIAFLEX?
- XIAFLEX should be injected into a cord by a healthcare provider who is experienced in injection procedures of the hand and treating people with Dupuytren’s contracture. If you have more than 1 contracture, your healthcare provider may give you 2 injections in 1 of your hands during your visit.
- Your healthcare provider will inject XIAFLEX into the cord that is causing your finger to bend.
- After an injection of XIAFLEX, your affected hand will be wrapped with a bandage. You should limit moving and using the treated finger after the injection.
- Do not bend or straighten the fingers of the injected hand until your healthcare provider says it is okay. This will help to keep the medicine from leaking out of the cord.
- Do not try to straighten the treated finger yourself.
- Keep the injected hand elevated until bedtime.
-
Call your healthcare provider right away if you have:
- signs of infection after your injection, such as fever, chills, increased redness, or swelling
- numbness or tingling in the treated finger
- trouble bending the injected finger after the swelling goes down
- Return to your healthcare provider’s office as directed 1 to 3 days after your injection. During this first follow-up visit, if you still have the cord, your healthcare provider may try to extend the treated finger to “break” the cord and try to straighten your finger.
- Your healthcare provider will provide you with a splint to wear on the treated finger. Wear the splint as instructed by your healthcare provider at bedtime to keep your finger straight.
- Do finger exercises each day, as instructed by your healthcare provider.
- Follow your healthcare provider’s instructions about when you can start doing your normal activities with the injected hand.
What are the possible side effects of XIAFLEX?
XIAFLEX may cause serious side effects, including:
- See “What is the most important information I should know about XIAFLEX?”
- increased chance of bleeding. Bleeding or bruising at the injection site can happen in people who receive XIAFLEX. Talk to your healthcare provider if you have a problem with your blood clotting. XIAFLEX may not be right for you.
The most common side effects with XIAFLEX for the treatment of Dupuytren’s contracture include:
- swelling of the injection site or the hand
- bruising or bleeding at the injection site
- pain or tenderness of the injection site or the hand
- swelling of the lymph nodes (glands) in the elbow or armpit (axilla)
- itching
- breaks in the skin
- redness or warmth of the skin
- pain in the armpit
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects with XIAFLEX. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of XIAFLEX.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
This Medication Guide summarizes the most important information about XIAFLEX. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about XIAFLEX that is written for health professionals.
For more information, go to www.XIAFLEX.com or call 1-800-462-3636.
What are the ingredients in XIAFLEX?
Active ingredient: collagenase clostridium histolyticum
Inactive ingredients: hydrochloric acid, sucrose, and tromethamine. The diluent contains: calcium chloride dihydrate in 0.9% sodium chloride
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Endo Pharmaceuticals Inc.
Malvern, PA 19355Manufactured by:
Auxilium Pharmaceuticals, LLC
Malvern, PA 19355US License No. 1816
US Patent Nos. 7,811,560 and RE39,941
Revised: 11/2019
-
SPL UNCLASSIFIED SECTION
Medication Guide
XIAFLEX®(Zī a flex)
(collagenase clostridium histolyticum)
For injection, for intralesional use
XIAFLEX is approved for two uses: Dupuytren's contracture and Peyronie's disease. Information is provided separately for each use. Use for treating Dupuytren's contracture is described on the previous pages, and use for treating Peyronie's disease is described below.
Read this Medication Guide before you receive XIAFLEX for the treatment of Peyronie’s disease and each time you get an injection. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment.
Your healthcare provider will also talk to you about receiving XIAFLEX for the treatment of Peyronie’s disease using the “What You Need to Know About XIAFLEX Treatment for Peyronie’s Disease: A Patient Guide”. You can ask your healthcare provider for a copy of the Patient Guide.
What is the most important information I should know about XIAFLEX for the treatment of Peyronie’s disease?
XIAFLEX can cause serious side effects, including:
-
Penile fracture (corporal rupture) or other serious injury to the penis. Receiving an injection of XIAFLEX may cause damage to the tubes in your penis called the corpora. After treatment with XIAFLEX, one of these tubes may break during an erection. This is called a corporal rupture or penile fracture. This could require surgery to fix the damaged area. Damage to your penis might not get better after a corporal rupture.
- After treatment with XIAFLEX, blood vessels in your penis may also break, causing blood to collect under the skin (hematoma). This could require a procedure to drain the blood from under the skin.
Symptoms of corporal rupture or other serious injury to your penis may include:- a popping sound or sensation in an erect penis
- sudden loss of the ability to maintain an erection
- pain in your penis
- purple bruising and swelling of your penis
- difficulty urinating or blood in the urine
Call your healthcare provider right away if you have any of the symptoms of corporal rupture or serious injury to the penis listed above.
Do not have sex or any other sexual activity between the first and second injections of a treatment cycle.
Do not have sex or have any other sexual activity for at least 4 weeks after the second injection of a treatment cycle with XIAFLEX and after any pain and swelling has gone away.
XIAFLEX for the treatment of Peyronie’s disease is only available through a restricted program called the XIAFLEX Risk Evaluation and Mitigation Strategy (REMS) Program. For more information about the XIAFLEX REMS Program go to www.XIAFLEXREMS.com or call 1-877-942-3539. - After treatment with XIAFLEX, blood vessels in your penis may also break, causing blood to collect under the skin (hematoma). This could require a procedure to drain the blood from under the skin.
-
Hypersensitivity reactions, including anaphylaxis. Severe allergic reactions can happen in people who receive XIAFLEX, because it contains foreign proteins.
Call your healthcare provider right away if you have any of these symptoms of an allergic reaction after an injection of XIAFLEX:- hives
- swollen face
- breathing trouble
- chest pain
- low blood pressure
- dizziness or fainting
What is XIAFLEX?
XIAFLEX is a prescription medicine used to treat adult men with Peyronie’s disease who have a “plaque” that can be felt and a curve in their penis greater than 30 degrees when treatment is started.
It is not known if XIAFLEX is safe and effective in children under the age of 18.
Who should not receive XIAFLEX?
Do not receive XIAFLEX if you:
- have been told by your healthcare provider that the Peyronie’s plaque to be treated involves the “tube” that your urine passes through (urethra).
- are allergic to collagenase clostridium histolyticum or any of the ingredients in XIAFLEX, or to any other collagenase product. See the end of this Medication Guide for a complete list of ingredients in XIAFLEX.
Talk to your healthcare provider before receiving this medicine if you have any of these conditions.
What should I tell my healthcare provider before receiving XIAFLEX?
Before receiving XIAFLEX, tell your healthcare provider if you:
- have had an allergic reaction to a XIAFLEX injection in the past
- have a bleeding problem
- have received XIAFLEX to treat another condition
- have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Using XIAFLEX with certain other medicines can cause side effects.
Especially tell your healthcare provider if you take:
- medicines to thin your blood (anticoagulants). If you are told to stop taking a blood thinner before your XIAFLEX injection, your healthcare provider should tell you when to start taking the blood thinner again.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How will I receive XIAFLEX?
- XIAFLEX should be injected into the plaque by a healthcare provider who is trained and experienced in treating adult men with Peyronie’s disease.
- Your healthcare provider will inject XIAFLEX into the plaque that is causing your penis to curve.
- XIAFLEX is given as part of a treatment cycle. In each treatment cycle, you will receive an injection of XIAFLEX, followed by a second injection 1 to 3 days later.
- After each injection of XIAFLEX, your penis may be wrapped with a bandage. Your healthcare provider will tell you when to take the bandage off.
- 1 to 3 days after your second injection of XIAFLEX in a treatment cycle, you will need to return to your healthcare provider’s office for a manual procedure that will stretch and help straighten your penis. Your healthcare provider will tell you when to come back for this.
- Your healthcare provider will show you how to gently stretch your penis the right way. See “How to gently stretch your penis.”
- You should gently stretch your penis 3 times a day for 6 weeks after each treatment cycle. You should only gently stretch your penis when you do not have an erection.
- Your healthcare provider will show you how to gently straighten your penis the right way. See “How to gently straighten your penis.”
- You should gently straighten your penis 1 time a day for 6 weeks after each treatment cycle. You should only gently straighten your penis if you have an erection that happens without any sexual activity (spontaneous erection).
- Do not use a vacuum erection device during your treatment with XIAFLEX.
- Your healthcare provider will tell you when you can resume sexual activity after each treatment cycle.
- Your healthcare provider will also tell you when to come back if more treatment cycles are needed.
Tell your healthcare provider right away if you have trouble stretching or straightening your penis, or if you have pain or other concerns.
How to gently stretch your penis:
Gently stretch your penis 3 times a day. Only stretch your penis if your penis is not hard (erect).
- With 1 hand, hold the tip of your penis with your fingers. With your other hand, hold the base of your penis with your fingers (See Figure A).
- Gently pull your penis away from your body to its full length and hold the stretch for 30 seconds.
- Let go of the tip of your penis and let your penis return to its normal length.
(Figure A)
How to gently straighten your penis:
Gently straighten your penis 1 time a day. Only straighten your penis if you have an erection that happens without any sexual activity (spontaneous erection). Bending your penis should not cause any pain or discomfort.
- With 1 hand hold your penis. With your other hand, gently bend your penis in the opposite direction of the curve (See Figure B). Hold the penis in this more straightened position for 30 seconds, then let go.
(Figure B)
What should I avoid while receiving XIAFLEX?
Avoid situations that may cause you to strain your stomach (abdominal) muscles, such as straining during bowel movements.What are the possible side effects of XIAFLEX?
XIAFLEX can cause serious side effects, including:
See “What is the most important information I should know about XIAFLEX?”
- increased chance of bleeding. Bleeding or bruising at the injection site can happen in people who receive XIAFLEX. Talk to your healthcare provider if you have a problem with your blood clotting. XIAFLEX may not be right for you.
The most common side effects with XIAFLEX for the treatment of Peyronie’s disease include:
- a small collection of blood under the skin at the injection site (hematoma)
- swelling at the injection site or along your penis
- pain or tenderness at the injection site, along your penis and above your penis
- penis bruising
- itching of your penis or scrotum (genitals)
- painful erection
- erection problems (erectile dysfunction)
- changes in the color of the skin of your penis
- blisters at the injection site
- pain with sex
- a lump at the injection site (nodule)
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects with XIAFLEX. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of XIAFLEX.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
This Medication Guide summarizes the most important information about XIAFLEX. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider for information about XIAFLEX that is written for health professionals.
For more information, go to www.XIAFLEX.com or call 1-800-462-3636.
What are the ingredients in XIAFLEX?
Active ingredient: collagenase clostridium histolyticum
Inactive ingredients: hydrochloric acid, sucrose, and tromethamine. The diluent contains: calcium chloride dihydrate in 0.9% sodium chloride
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Endo Pharmaceuticals Inc.
Malvern, PA 19355Manufactured by:
Auxilium Pharmaceuticals, LLC
Malvern, PA 19355US License No. 1816
US Patent Nos. 7,811,560 and 6,022,539
Revised: 11/2019
-
Penile fracture (corporal rupture) or other serious injury to the penis. Receiving an injection of XIAFLEX may cause damage to the tubes in your penis called the corpora. After treatment with XIAFLEX, one of these tubes may break during an erection. This is called a corporal rupture or penile fracture. This could require surgery to fix the damaged area. Damage to your penis might not get better after a corporal rupture.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
XIAFLEX
collagenase clostridium histolyticum kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66887-003 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66887-003-02 2 in 1 CARTON 02/22/2010 1 1 in 1 CARTON; Type 0: Not a Combination Product 2 NDC: 66887-003-01 1 in 1 CARTON; Type 0: Not a Combination Product 02/22/2010 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 Part 2 1 VIAL 3 mL Part 1 of 2 XIAFLEX
collagenase clostridium histolyticum injection, powder, lyophilized, for solutionProduct Information Route of Administration INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COLLAGENASE CLOSTRIDIUM HISTOLYTICUM (UNII: 9X7O8V25IT) (COLLAGENASE CLOSTRIDIUM HISTOLYTICUM - UNII:9X7O8V25IT) COLLAGENASE CLOSTRIDIUM HISTOLYTICUM 0.9 mg Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) 0.5 mg SUCROSE (UNII: C151H8M554) 18.5 mg TROMETHAMINE (UNII: 023C2WHX2V) 1.1 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125338 02/22/2010 Part 2 of 2 STERILE DILUENT
diluent solutionProduct Information Route of Administration INTRALESIONAL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.9 mg in 3 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125338 02/22/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125338 02/22/2010 Labeler - Endo Pharmaceuticals Inc. (178074951) Establishment Name Address ID/FEI Business Operations Auxilium Pharmaceuticals, LLC 801535787 ANALYSIS(66887-003) , API MANUFACTURE(66887-003) Establishment Name Address ID/FEI Business Operations Charles River Biopharmaceutical Services GmbH 344516158 ANALYSIS(66887-003) Establishment Name Address ID/FEI Business Operations Eurofins Lancaster Laboratories, Inc. 069777290 ANALYSIS(66887-003) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier LLC 069263643 ANALYSIS(66887-003) , MANUFACTURE(66887-003) Establishment Name Address ID/FEI Business Operations KBI BioPharma, Inc. 034248380 ANALYSIS(66887-003) Establishment Name Address ID/FEI Business Operations Nelson Laboratories, Inc. 151663234 ANALYSIS(66887-003) Establishment Name Address ID/FEI Business Operations Packaging Coordinators, Inc. 078525133 MANUFACTURE(66887-003) Establishment Name Address ID/FEI Business Operations Patheon Manufacturing Services LLC 079415560 MANUFACTURE(66887-003) Establishment Name Address ID/FEI Business Operations West Pharmaceutical Services, Inc. 002330983 ANALYSIS(66887-003)
Trademark Results [XIAFLEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XIAFLEX 77274256 3886633 Live/Registered |
ENDO GLOBAL VENTURES 2007-09-07 |
 XIAFLEX 76665957 3785074 Live/Registered |
ENDO GLOBAL VENTURES 2006-09-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.