LYMPHOMYOSOT X- araneus diadematus, tribasic calcium phosphate, equisetum hyemale, ferrous iodide,fumaria officinalis flowering top, gentiana lutea root, geranium robertianum, myosotis arvensis, nasturtium officinale, sodium sulfate, pinus sylvestris leafy twig,smilax regelii root, scrophularia nodosa, teucrium scorodonia flowering top, thyroid, unspecified andveronica officinalis flowering top injection
Lymphomyosot X by
Drug Labeling and Warnings
Lymphomyosot X by is a Homeopathic medication manufactured, distributed, or labeled by MediNatura, Hameln Pharma GmbH, Biologische Heilmittel Heel. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Ingredient name
Potency
Quantity
Final dilution
Aranea diadema
6X
0.55 μl
9.30X
Calcarea phosphorica
12X
0.55 μl
15.30X
Equisetum hyemale
4X
0.55 μl
7.30X
Ferrum iodatum
12X
1.1 μl
15.00X
Fumaria officinalis
4X
0.55 μl
7.30X
Gentiana lutea
5X
0.55 μl
8.30X
Geranium robertianum
4X
1.1 μl
7.00X
Myosotis arvensis
3X
0.55 μl
6.30X
Nasturtium aquaticum
4X
1.1 μl
7.00X
Natrum sulphuricum
4X
0.55 μl
7.30X
Pinus sylvestris
4X
0.55 μl
7.30X
Sarsaparilla
6X
0.55 μl
9.30X
Scrophularia nodosa
3X
0.55 μl
6.30X
Teucrium scorodonia
3X
0.55 μl
6.30X
Thyroidinum
12X
0.55 μl
15.30X
Veronica officinalis
3X
0.55 μl
6.30X
- INDICATION AND USAGE
-
DOSAGE AND ADMINISTRATION
General Consideration
If co-administration with a local anesthetic is desired, Lymphomyosot® X Injection Solution may be mixed with lidocaine or similar agents at the discretion of the physician.
The dosage schedules listed below can be used as a general guide for the administration of Lymphomyosot® X Injection Solution.
Lymphomyosot® X Injection Solution may be administered s.c., i.d., i.m., or i.v.
The interval between injections is left to the discretion of the HCP, but should not exceed 1 ampule in 24 hours.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to
administration, whenever solution and container permit.
Draw up required dose into syringe.
Discard any unused ampule contents. Do not reuse ampule.
Only licensed practitioners with sufficient expertise in injecting drugs, including the respective route of administration, should administer the product.
2.2 Standard Dosage:
Adults and children 12 years and older:
1 ml 1 to 3 times per 7 days.
Children 6 to 11 years:
0.7 ml 1 to 3 times per 7 days.
Children 2 to 5 years:
0.5 ml 1 to 3 times per 7 days.
2.3 Acute Dosage:
Adults and children 12 years and older:
1 ml daily, and then continue with standard dosage.
Children 6 to 11 years:
0.7 ml daily, and then continue with standard dosage.
Children 2 to 5 years:
0.5 ml daily, and then continue with standard dosage.
- WARNINGS AND PRECAUTIONS
- CONTRAINDICATIONS
- OVERDOSAGE
-
ADVERSE REACTIONS
Post-marketing Experience
The following adverse events have been identified during post-marketing use of Lymphomyosot® X Injection Solution. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure
Allergic (hypersensitivity) skin reactions may occur in isolated cases.
To report SUSPECTED ADVERSE REACTIONS, contact MediNatura. at 1.844.633.4628 or info@medinatura.com or FDA at 1-800-FDA-1088
- DOSAGE FORMS AND STRENGTH
- MECHANISM OF ACTION
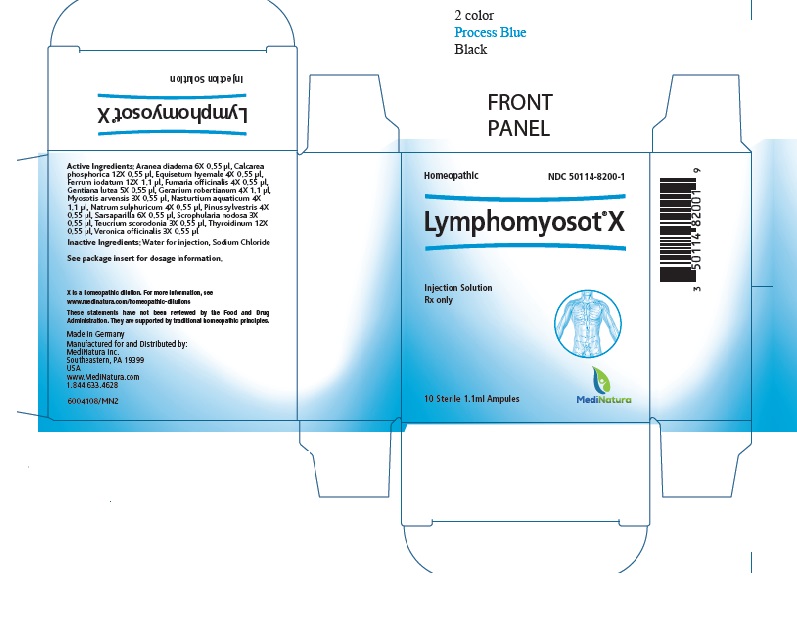
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LYMPHOMYOSOT X
araneus diadematus, tribasic calcium phosphate, equisetum hyemale, ferrous iodide,fumaria officinalis flowering top, gentiana lutea root, geranium robertianum, myosotis arvensis, nasturtium officinale, sodium sulfate, pinus sylvestris leafy twig,smilax regelii root, scrophularia nodosa, teucrium scorodonia flowering top, thyroid, unspecified andveronica officinalis flowering top injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50114-8200 Route of Administration INTRADERMAL, INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARANEUS DIADEMATUS (UNII: 6T6CO7R3Z5) (ARANEUS DIADEMATUS - UNII:6T6CO7R3Z5) ARANEUS DIADEMATUS 6 [hp_X] in 1.1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] in 1.1 mL EQUISETUM HYEMALE (UNII: 59677RXH25) (EQUISETUM HYEMALE - UNII:59677RXH25) EQUISETUM HYEMALE 12 [hp_X] in 1.1 mL FERROUS IODIDE (UNII: F5452U54PN) (FERROUS IODIDE - UNII:F5452U54PN) FERROUS IODIDE 3 [hp_X] in 1.1 mL FUMARIA OFFICINALIS FLOWERING TOP (UNII: VH659J61ZL) (FUMARIA OFFICINALIS FLOWERING TOP - UNII:VH659J61ZL) FUMARIA OFFICINALIS FLOWERING TOP 4 [hp_X] in 1.1 mL GENTIANA LUTEA ROOT (UNII: S72O3284MS) (GENTIANA LUTEA ROOT - UNII:S72O3284MS) GENTIANA LUTEA ROOT 5 [hp_X] in 1.1 mL GERANIUM ROBERTIANUM (UNII: R5I1HK0UBL) (GERANIUM ROBERTIANUM - UNII:R5I1HK0UBL) GERANIUM ROBERTIANUM 4 [hp_X] in 1.1 mL MYOSOTIS ARVENSIS (UNII: C73BK97H5J) (MYOSOTIS ARVENSIS - UNII:C73BK97H5J) MYOSOTIS ARVENSIS 3 [hp_X] in 1.1 mL NASTURTIUM OFFICINALE (UNII: YH89GMV676) (NASTURTIUM OFFICINALE - UNII:YH89GMV676) NASTURTIUM OFFICINALE 4 [hp_X] in 1.1 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 4 [hp_X] in 1.1 mL PINUS SYLVESTRIS LEAFY TWIG (UNII: Q1RGP4UB73) (PINUS SYLVESTRIS LEAFY TWIG - UNII:Q1RGP4UB73) PINUS SYLVESTRIS LEAFY TWIG 4 [hp_X] in 1.1 mL SMILAX ORNATA ROOT (UNII: 2H1576D5WG) (SMILAX ORNATA ROOT - UNII:2H1576D5WG) SMILAX ORNATA ROOT 6 [hp_X] in 1.1 mL SCROPHULARIA NODOSA (UNII: 7H443NUB2T) (SCROPHULARIA NODOSA - UNII:7H443NUB2T) SCROPHULARIA NODOSA 3 [hp_X] in 1.1 mL TEUCRIUM SCORODONIA FLOWERING TOP (UNII: LOK3I16O7G) (TEUCRIUM SCORODONIA FLOWERING TOP - UNII:LOK3I16O7G) TEUCRIUM SCORODONIA FLOWERING TOP 3 [hp_X] in 1.1 mL THYROID, UNSPECIFIED (UNII: 0B4FDL9I6P) (THYROID, UNSPECIFIED - UNII:0B4FDL9I6P) THYROID, UNSPECIFIED 12 [hp_X] in 1.1 mL VERONICA OFFICINALIS FLOWERING TOP (UNII: 9IH82J936J) (VERONICA OFFICINALIS FLOWERING TOP - UNII:9IH82J936J) VERONICA OFFICINALIS FLOWERING TOP 3 [hp_X] in 1.1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50114-8200-3 3 in 1 CARTON 07/31/2014 1 1.1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 50114-8200-1 10 in 1 CARTON 07/31/2014 2 1.1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/31/2014 Labeler - MediNatura (102783016) Establishment Name Address ID/FEI Business Operations Hameln Pharma GmbH 315869123 manufacture(50114-8200) Establishment Name Address ID/FEI Business Operations Biologische Heilmittel Heel 315635359 manufacture(50114-8200)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.