BLUJEPA- gepotidacin tablet, film coated
Blujepa by
Drug Labeling and Warnings
Blujepa by is a Prescription medication manufactured, distributed, or labeled by GlaxoSmithKline LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BLUJEPA safely and effectively. See full prescribing information for BLUJEPA.
BLUJEPA (gepotidacin) tablets, for oral use
Initial U.S. Approval: 2025RECENT MAJOR CHANGES
INDICATIONS AND USAGE
BLUJEPA is a triazaacenaphthylene bacterial type II topoisomerase inhibitor indicated for the treatment of the following infections caused by susceptible microorganisms:
- Uncomplicated urinary tract infections (uUTI) in female adult and pediatric patients 12 years of age and older weighing at least 40 kilograms (kg). (1.1)
- Uncomplicated urogenital gonorrhea in adult and pediatric patients 12 years of age and older weighing at least 45 kilograms who have limited or no alternative treatment options. Approval of this indication is based on limited clinical safety data for this indication. (1.2, 6.1)
Usage to Reduce Development of Drug-Resistant Bacteria
To reduce the development of drug-resistant bacteria and maintain the effectiveness of BLUJEPA and other antibacterial drugs, BLUJEPA should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. (1.3)
DOSAGE AND ADMINISTRATION
- uUTI: The recommended dosage of BLUJEPA is 1,500 mg (two 750 mg tablets) taken orally, twice daily (approximately 12 hours apart), for 5 days. (2.1)
- Uncomplicated Urogenital Gonorrhea: The recommended dosage of BLUJEPA is 3,000 mg (four 750 mg tablets) taken orally, followed by a second dose of 3,000 mg (four 750 mg tablets) approximately 12 hours later. (2.2)
- Administer BLUJEPA tablets after a meal to reduce the possibility of gastrointestinal intolerance. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 750 mg of gepotidacin. (3)
CONTRAINDICATIONS
A history of severe hypersensitivity to BLUJEPA. (4)
WARNINGS AND PRECAUTIONS
- QTc Prolongation:
- Avoid use of BLUJEPA in patients with a history of QTc prolongation, or with relevant pre‑existing cardiac disease, or in patients receiving drugs that prolong the QTc interval. (5.1)
- Due to an increase in gepotidacin exposure and the risk of QTc interval prolongation, avoid use of BLUJEPA in patients who have any of the following risk factors: (5.1, 7.1, 8.6, 8.7)
- Concomitant use of strong CYP3A4 inhibitors
- Severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min)
- Severe hepatic impairment (Child-Pugh Class C)
- Additionally, avoid BLUJEPA in uncomplicated urogenital gonorrhea patients, who have any of the following risk factors for increased gepotidacin exposure: (5.1, 7.1, 8.6, 8.7)
- Concomitant use of moderate CYP3A4 inhibitors
- Two or more of the following risk factors: Body weight between 45 kilograms and 60 kilograms, Moderate renal impairment (eGFR 30 to 59 mL/min), Moderate hepatic impairment (Child-Pugh Class B)
- Acetylcholinesterase Inhibition: Dysarthria and other adverse reactions have been reported in patients receiving BLUJEPA. Monitor patients with underlying medical conditions that may be exacerbated by acetylcholinesterase inhibition and patients receiving succinylcholine‑type neuromuscular blocking agents, systemic anticholinergic medications, or non‑depolarizing neuromuscular blocking agents. (5.2)
- Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, have been reported in patients receiving BLUJEPA. If an allergic reaction to BLUJEPA occurs, discontinue the drug and institute appropriate supportive measures. (5.3)
- Clostridioides difficile Infection (CDI): CDI has been reported with nearly all systemic antibacterial agents, including BLUJEPA. Evaluate patients who develop diarrhea. (5.4)
ADVERSE REACTIONS
- uUTI: The most common adverse reactions occurring in ≥1% of patients are diarrhea, nausea, abdominal pain, flatulence, headache, soft feces, dizziness, vomiting, and vulvovaginal candidiasis. (6.1)
- Uncomplicated Urogenital Gonorrhea: The most common adverse reactions occurring in ≥2% of patients are diarrhea, nausea, abdominal pain, vomiting, flatulence, dizziness, soft feces, headache, fatigue, and hyperhidrosis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP3A4 Inhibitors: Increase gepotidacin exposure. (7.1)
- Strong CYP3A4 inhibitors: Avoid concomitant use of BLUJEPA with strong CYP3A4 inhibitors. (5.1, 7.1)
- Moderate CYP3A4 inhibitors: Avoid concomitant use of BLUJEPA with moderate CYP3A4 inhibitors in patients with uncomplicated urogenital gonorrhea. (5.1, 7.1)
- CYP3A4 Inducers: Decrease gepotidacin exposure. (7.1)
- For uUTI: Avoid concomitant use of BLUJEPA with strong CYP3A4 inducers. (7.1)
- For uncomplicated urogenital gonorrhea: Avoid concomitant use of BLUJEPA with strong and moderate CYP3A4 inducers. (7.1)
- CYP3A4 Substrates: Avoid concomitant use of BLUJEPA with drugs that are extensively metabolized by CYP3A4 where minimal concentration changes may lead to serious adverse reactions. (7.2)
- Digoxin: Due to an increase in digoxin exposures, consider monitoring digoxin serum concentration, as appropriate, with concomitant administration of BLUJEPA. (7.2)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Uncomplicated Urinary Tract Infections
1.2 Treatment of Uncomplicated Urogenital Gonorrhea
1.3 Usage to Reduce Development of Drug-Resistant Bacteria
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Female Adult and Pediatric Patients 12 Years of Age and Older Weighing at Least 40 kg for Uncomplicated UTI
2.2 Recommended Dosage for Adult and Pediatric Patients 12 Years of Age and Older Weighing at Least 45 kg for Uncomplicated Urogenital Gonorrhea
2.3 Important Administration Instructions
2.4 Recommendations Regarding Missed Dose(s)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 QTc Prolongation
5.2 Acetylcholinesterase Inhibition
5.3 Hypersensitivity Reactions
5.4 Clostridioides difficile Infection
5.5 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on BLUJEPA
7.2 Effect of BLUJEPA on Other Drugs
7.3 Cholinergic/Anticholinergic Drugs
7.4 Drugs that Prolong the QTc Interval
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Uncomplicated Urinary Tract Infections
14.2 Uncomplicated Urogenital Gonorrhea
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Treatment of Uncomplicated Urinary Tract Infections
BLUJEPA is indicated in female adult and pediatric patients 12 years of age and older weighing at least 40 kilograms (kg) for the treatment of uncomplicated urinary tract infections (uUTI) caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii complex, Staphylococcus saprophyticus, and Enterococcus faecalis.
1.2 Treatment of Uncomplicated Urogenital Gonorrhea
BLUJEPA is indicated in adult and pediatric patients 12 years of age and older weighing at least 45 kilograms (kg) who have limited or no alternative options for the treatment of uncomplicated urogenital gonorrhea caused by susceptible strains of Neisseria gonorrhoeae. Approval of this indication is based on limited clinical safety data for BLUJEPA [see Adverse Reactions (6.1)].
1.3 Usage to Reduce Development of Drug-Resistant Bacteria
To reduce the development of drug-resistant bacteria and maintain the effectiveness of BLUJEPA and other antibacterial drugs, BLUJEPA should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Female Adult and Pediatric Patients 12 Years of Age and Older Weighing at Least 40 kg for Uncomplicated UTI
The recommended dosage of BLUJEPA is 1,500 mg (two 750 mg tablets) taken orally, twice daily (approximately 12 hours apart) for 5 days in female adult and pediatric patients 12 years of age and older with uncomplicated uUTI [see Dosage and Administration (2.3)].
2.2 Recommended Dosage for Adult and Pediatric Patients 12 Years of Age and Older Weighing at Least 45 kg for Uncomplicated Urogenital Gonorrhea
The recommended dose of BLUJEPA is 3,000 mg (four 750 mg tablets) taken orally, followed by a second dose of 3,000 mg (four 750 mg tablets) approximately 12 hours later in adult and pediatric patients 12 years of age and older weighing at least 45 kg with uncomplicated urogenital gonorrhea [see Dosage and Administration (2.3)].
Do not increase the dose, extend the duration of treatment, or reduce the interval between doses due to the risk of QTc interval prolongation [see Warnings and Precautions (5.1)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 QTc Prolongation
A dose and concentration-dependent prolongation of the QTc interval has been observed with BLUJEPA [see Clinical Pharmacology (12.2)].
Avoid BLUJEPA in patients with a history of QTc interval prolongation or those with relevant pre‑existing cardiac disease, patients taking antiarrhythmic agents, or other medications that may potentially prolong the QTc interval [see Drug Interactions (7.4)].
Due to an increase in gepotidacin exposure (Cmax), and the risk of QTc interval prolongation, avoid BLUJEPA in patients who have any of the following risk factors [see Drug Interactions (7.1), Use in Specific Populations (8.6, 8.7)]:
- Concomitant use of strong CYP3A4 inhibitors
- Severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min)
- Severe hepatic impairment (Child-Pugh Class C)
Additionally, avoid BLUJEPA in uncomplicated urogenital gonorrhea patients who also have any of the following risk factors for increased gepotidacin exposure [see Drug Interactions (7.1), Use in Specific Populations (8.6, 8.7)]:
- Concomitant use of moderate CYP3A4 inhibitors
-
Two or more of the following risk factors:
- o Body weight between 45 kg and 60 kg
- o Moderate renal impairment (eGFR 30 to 59 mL/min)
- o Moderate hepatic impairment (Child‑Pugh Class B)
If administration of BLUJEPA cannot be avoided in these patients, monitor and correct serum electrolyte abnormalities and collect an ECG prior to administration and during treatment, as clinically indicated.
5.2 Acetylcholinesterase Inhibition
BLUJEPA is a reversible acetylcholinesterase inhibitor in in vitro laboratory studies. Adverse reactions including dysarthria, syncope, presyncope, muscle spasms, diarrhea, nausea, vomiting, abdominal pain, hypersalivation, and hyperhidrosis which are potentially attributed to acetylcholinesterase inhibition, have been observed in clinical trials [see Adverse Reactions (6.1)]. Increased cholinergic effects can be associated with severe adverse reactions including atrioventricular block, bradycardia, bronchospasm, and seizures/convulsions. Monitor patients with medical conditions that may be exacerbated by acetylcholinesterase inhibition.
BLUJEPA, as an acetylcholinesterase inhibitor, may exaggerate the neuromuscular effects of succinylcholine‑type muscle relaxation during anesthesia. BLUJEPA may exaggerate the effects of other acetylcholinesterase inhibitors. Monitor patients for exaggerated neuromuscular blockade or excessive cholinergic effects.
Because BLUJEPA may antagonize the effects of systemic anticholinergic medications or non‑depolarizing neuromuscular blocking agents, monitor patients if BLUJEPA is concomitantly administered with these medications [see Drug Interactions (7.3)].
5.3 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have been reported in patients receiving BLUJEPA [see Adverse Reactions (6.1)]. BLUJEPA is contraindicated in patients with a history of severe hypersensitivity to BLUJEPA [see Contraindications (4)]. Before therapy with BLUJEPA is instituted, carefully inquire about previous hypersensitivity reactions to BLUJEPA. If an allergic reaction to BLUJEPA occurs, discontinue the drug and institute appropriate supportive measures.
5.4 Clostridioides difficile Infection
Clostridioides difficile (C. difficile) infection (CDI) has been reported for nearly all systemic antibacterial agents, including BLUJEPA, and may range in severity from mild diarrhea to fatal colitis [see Adverse Reactions (6)]. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDI. Hypertoxin producing isolates of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDI must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDI has been reported to occur over 2 months after the administration of antibacterial agents.
If CDI is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.5 Development of Drug-Resistant Bacteria
Prescribing BLUJEPA in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug‑resistant bacteria [see Indications and Usage (1.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- QTc Prolongation [see Warnings and Precautions (5.1)].
- Acetylcholinesterase Inhibition [see Warnings and Precautions (5.2)].
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)].
- Clostridioides difficile Infection [see Warnings and Precautions (5.4)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Patients with Uncomplicated UTI
The safety of BLUJEPA was evaluated in 2 double‑blind, active‑controlled, randomized trials in female adult and pediatric patients 12 years of age and older with uUTI (Trial 1 and Trial 2). A total of 1,570 patients were treated with BLUJEPA and 1,558 patients were treated with nitrofurantoin (pooled safety populations for BLUJEPA and nitrofurantoin, respectively). Patients received treatment for a median duration of 5 days.
In Trials 1 and 2 (pooled, intent-to-treat [ITT] population), the median age of patients treated with BLUJEPA was 49 (range 13 to 89) years; <1% were <18 years, 77% of patients were 18 to 64 years, 14% were 65 to 74 years, and 8% were ≥75 years. Patients were female (100%) and White (83%), Black or African American (7%), Asian (5%), or American Indian or Alaskan Native (4%); for ethnicity, 33% identified as Hispanic/Latino and 67% as non-Hispanic/Latino. The majority of patients were enrolled from the U.S. (55%).
Serious Adverse Reactions and Adverse Reactions Leading to Discontinuation: In the pooled trials (Trials 1 and 2), serious adverse reactions occurred in 1/1,570 (<1%) uUTI patients treated with BLUJEPA and 1/1,558 (<1%) uUTI patients treated with nitrofurantoin. The serious adverse reaction reported with BLUJEPA was dysarthria. No adverse reaction led to death in either treatment group.
In the pooled trials, adverse reactions leading to discontinuation of treatment occurred in 79/1,570 (5%) uUTI patients treated with BLUJEPA and 30/1,558 (2%) uUTI patients treated with nitrofurantoin. Adverse reactions occurring in >1% of patients leading to treatment discontinuation in patients treated with BLUJEPA included diarrhea (3%) and nausea (1%).
Common Adverse Reactions: Table 1 lists the adverse reactions occurring in ≥1% of uUTI patients receiving BLUJEPA in the pooled trials (Trials 1 and 2).
Table 1. Adverse Reactions Occurring in ≥1% of Uncomplicated Urinary Tract Infection Patients Treated with BLUJEPA (Trials 1 and 2 Pooled Data; Safety Population) a Abdominal pain includes abdominal pain, abdominal pain upper, abdominal pain lower, abdominal tenderness, abdominal discomfort, and gastrointestinal pain. Adverse Reaction
BLUJEPA
N = 1,570
n (%)
Nitrofurantoin
N = 1,558
n (%)
Diarrhea
258 (16)
51 (3)
Nausea
146 (9)
64 (4)
Abdominal paina
60 (4)
34 (2)
Flatulence
43 (3)
8 (<1)
Headache
38 (2)
40 (3)
Soft feces
37 (2)
8 (<1)
Dizziness
29 (2)
19 (1)
Vomiting
28 (2)
10 (<1)
Vulvovaginal candidiasis
20 (1)
18 (1)
Diarrhea: In Trials 1 and 2, diarrhea was reported in 258/1,570 (16%) uUTI patients receiving BLUJEPA; 11% mild, 5% moderate, and <1% severe. The diarrhea started within the first 2 days of treatment for the majority of patients and the median duration of diarrhea was 4 days.
Adverse Reactions Occurring in Less than 1% of uUTI Patients Receiving BLUJEPA in Trials 1 and 2 (Pooled):
Gastrointestinal Disorders: Abdominal distension, dyspepsia (includes epigastric discomfort, eructation)
Nervous System Disorders: Presyncope, dysarthria
Infections and Infestations: Clostridioides difficile infection
Musculoskeletal and Connective Tissue Disorders: Muscle spasms
Vascular Disorders: Hot flush
Cardiac Disorders: Tachycardia
Eye Disorders: Blurred vision
Ear and Labyrinth Disorders: Vertigo
General Disorders and Administration Site Disorders: Fatigue
Investigations: Alanine aminotransferase/aspartate aminotransferase increased
Skin and Subcutaneous Tissue: Rash, hyperhidrosis
Immune System Disorders: Hypersensitivity reactions
Select Adverse Reactions Occurring in uUTI Patients Receiving BLUJEPA in Phase 1 and 2 Clinical Studies: Gastrointestinal Disorders: Hypersalivation (with oral daily doses ranging from 100 mg to 6,000 mg, which includes not approved doses)
Clinical Trial Experience in Patients with Uncomplicated Urogenital Gonorrhea
The safety of BLUJEPA was evaluated in a randomized, active‑controlled trial (Trial 3) comparing BLUJEPA to ceftriaxone and azithromycin in adult and pediatric patients 12 years of age and older with uncomplicated urogenital gonorrhea. A total of 309 patients received at least one dose of BLUJEPA (safety population).
In Trial 3 (ITT population), the median age of patients randomized to receive BLUJEPA was 33 (range 16 to 64) years; >99% of patients were 18 to 65 years (no patients were >65 years). Overall, patients randomized to receive BLUJEPA treatment were predominately male (89%) and White (74%).
Serious Adverse Reactions and Adverse Reactions Leading to Discontinuation: No serious adverse reactions occurred in uncomplicated urogenital gonorrhea patients treated with BLUJEPA in Trial 3. Adverse reactions leading to discontinuation of treatment occurred in 3/309 (<1%) of uncomplicated urogenital gonorrhea patients treated with BLUJEPA.
Common Adverse Reactions: Table 2 lists the adverse reactions occurring in ≥2% of uncomplicated urogenital gonorrhea patients receiving BLUJEPA in Trial 3.
Table 2. Adverse Reactions Occurring in ≥2% of Uncomplicated Urogenital Gonorrhea Patients Treated with BLUJEPA (Trial 3; Safety Population) a Abdominal pain includes abdominal pain, abdominal pain upper, and abdominal discomfort. b Fatigue includes fatigue and lethargy. Adverse Reaction
BLUJEPA
N = 309
n (%)
Ceftriaxone and Azithromycin
N = 313
n (%)
Diarrhea
151 (49)
30 (10)
Nausea
73 (24)
9 (3)
Abdominal paina
25 (8)
6 (2)
Vomiting
20 (6)
2 (<1)
Flatulence
20 (6)
1 (<1)
Dizziness
16 (5)
2 (<1)
Soft feces
16 (5)
1 (<1)
Headache
10 (3)
8 (3)
Fatigueb
10 (3)
0
Hyperhidrosis
7 (2)
0
Diarrhea: In Trial 3, diarrhea was reported in 151/309 (49%) uncomplicated urogenital gonorrhea patients receiving BLUJEPA (38% mild severity, 11% moderate severity). Most episodes of diarrhea started on the same day as dosing, with few diarrhea episodes after day 2. The median duration was 2 days. Discontinuation of BLUJEPA due to diarrhea was reported in 1/309 (<1%) uncomplicated urogenital gonorrhea patients.
Adverse Reactions Occurring in Less than 2% of Uncomplicated Urogenital Gonorrhea Patients Receiving BLUJEPA in Trial 3:
Skin and Subcutaneous Tissue: Rash
Cardiac Disorders: Tachycardia
Nervous System Disorders: Dysarthria, presyncope, syncope
Musculoskeletal and Connective Tissue Disorders: Muscle spasms, myalgia, arthralgia
Vascular Disorders: Hot flush
Gastrointestinal Disorders: Hypersalivation, abdominal distension
Eye Disorders: Vision blurred
Ear and Labyrinth Disorders: Vertigo
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on BLUJEPA
CYP3A4 Inhibitors
Table 3. Recommendations for Concomitant Administration of CYP3A4 Inhibitors with BLUJEPA Indication
Moderate CYP3A4 Inhibitors
Strong CYP3A4 Inhibitors
Uncomplicated UTI
No dosage adjustment
Avoid concomitant administration of BLUJEPA with strong inhibitors of CYP3A4 due to an increase in gepotidacin exposure [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
Uncomplicated Urogenital Gonorrhea
Avoid concomitant administration of BLUJEPA with moderate CYP3A4 inhibitors due to an increase in gepotidacin exposure [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
Avoid concomitant administration of BLUJEPA with strong inhibitors of CYP3A4 due to an increase in gepotidacin exposure [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
CYP3A4 Inducers
Due to decreased gepotidacin exposures, avoid coadministration of BLUJEPA with CYP3A4 inducers as follows:
7.2 Effect of BLUJEPA on Other Drugs
CYP3A4 Substrates
Avoid concomitant administration of BLUJEPA with drugs that are extensively metabolized by CYP3A4 where minimal concentration changes may lead to serious adverse reactions [see Clinical Pharmacology (12.3)].
Digoxin
Due to an increase in digoxin exposures, consider monitoring digoxin serum concentrations, as appropriate, with concomitant administration of BLUJEPA [see Clinical Pharmacology (12.3)].
7.3 Cholinergic/Anticholinergic Drugs
As gepotidacin is an acetylcholinesterase inhibitor, there is potential for an exaggerated effect of concomitantly administered succinylcholine‑type neuromuscular blocking agents resulting in a delay in recovery of neuromuscular function. BLUJEPA may augment the effect of other acetylcholinesterase inhibitors. Monitor for exaggerated neuromuscular blockade or excessive cholinergic effects [see Warnings and Precautions (5.2)].
There is potential for an antagonistic effect with systemic anticholinergic medications or non‑depolarizing neuromuscular blocking agents. Consider the potential for this interaction if BLUJEPA is administered concomitantly with anticholinergic medications [see Warnings and Precautions (5.2)].
7.4 Drugs that Prolong the QTc Interval
Due to the increased risk of QTc prolongation, avoid concomitant administration of BLUJEPA with other medications that have the potential to prolong the QTc interval [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
A pregnancy exposure registry will be established to monitor pregnancy outcomes in women exposed to BLUJEPA during pregnancy. Pregnant women exposed to BLUJEPA, and healthcare providers are encouraged to contact GlaxoSmithKline at 1‑888‑825‑5249.
Risk Summary
There are no available data on the use of BLUJEPA in pregnant women to evaluate for a drug‑associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
In embryo‑fetal development studies in mice and rats, decreased fetal weights and increased fetal mortality (late resorptions) were observed at exposures less than the maximum recommended human dose (MRHD). In a mouse pre- and postnatal development study, there were no adverse developmental effects at exposures approximately equal to the MRHD (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage, in clinically recognized pregnancies, is 2% to 4% and 15% to 20%, respectively.
Animal Data: Gepotidacin did not cause malformations when orally administered in embryo‑fetal development studies during organogenesis. Decreased fetal weights were seen in rats dosed orally with 450 mg/kg/day or greater (less than the MRHD based on AUC extrapolated from nonpregnant rats). Decreased fetal weights and increased late fetal resorptions were seen in mice at oral doses 500 mg/kg/day or greater (less than the MRHD based on AUC extrapolated from nonpregnant mice).
In a pre- and post‑natal development study in mice given oral doses of up to 1,000 mg/kg/day (approximately equal to the MRHD), there were no gepotidacin effects on parturition, or post‑natal growth and development of the offspring.
8.2 Lactation
Risk Summary
There are no data on the presence of gepotidacin in human milk, its effects on the breastfed child, or on milk production. Based on a study in lactating mice, gepotidacin is likely transferred into milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BLUJEPA and any potential adverse effects on the breastfed child from BLUJEPA or from the underlying maternal condition.
Gepotidacin concentrations in milk have not been measured directly in animals. However, in a pre- and post‑natal development study when gepotidacin was orally administered to maternal mice during pregnancy and lactation, at all tested doses, gepotidacin was detected in the plasma of their nursing offspring (with dosing 200 mg/kg/day or greater, which was expected to result in maternal exposures as low as 0.2-times the MHRD as extrapolated from exposures in nonpregnant mice).
8.4 Pediatric Use
Uncomplicated UTI
The safety and effectiveness of BLUJEPA for the treatment of uUTI have been established for the treatment of uncomplicated UTI in female pediatric patients 12 years of age and older, weighing at least 40 kg. Use of BLUJEPA in these patients is supported by evidence from adequate and well‑controlled studies of BLUJEPA in female adult and pediatric patients 12 years of age and older with uUTI and additional pharmacokinetic data in pediatric patients (12 to <18 years of age) [see Clinical Pharmacology (12.3), Clinical Studies (14.1)]. The safety profile of BLUJEPA in female pediatric patients 12 years of age and older was similar to female adults with uUTI treated with BLUJEPA [see Adverse Reactions (6.1), Clinical Studies (14.1)].
The safety and effectiveness of BLUJEPA have not been established in pediatric patients less than 12 years of age or weighing less than 40 kg.
Uncomplicated Urogenital Gonorrhea
The safety and effectiveness of BLUJEPA for the treatment of uncomplicated urogenital gonorrhea have been established in pediatric patients 12 years of age and older weighing at least 45 kg who have limited or no alternative treatment options [see Indications and Usage (1.1)]. Use of BLUJEPA in these patients is supported by evidence from an adequate and well‑controlled study (Trial 3) in adult and pediatric patients with uncomplicated urogenital gonorrhea weighing at least 45 kg, and additional pharmacokinetic analyses showing similar drug exposure levels for pediatric patients (12 to <18 years of age) compared with adults [see Clinical Pharmacology (12.3), Clinical Studies (14.2)]. The safety profile of BLUJEPA was similar between pediatric healthy volunteers 12 to <18 years of age (n = 12) who received two 3,000 mg doses compared to adults [see Adverse Reactions (6.1)].
The safety and effectiveness of BLUJEPA have not been established for the treatment of uncomplicated urogenital gonorrhea in pediatric patients less than 12 years of age or weighing less than 45 kg.
8.5 Geriatric Use
Uncomplicated UTI
Of the total number of patients who received treatment with BLUJEPA in the uUTI studies (Trials 1 and 2), 226 (14%) were 65 to less than 75 years of age and 127 (8%) were 75 years of age and older [see Clinical Studies (14.1)]. No overall differences in safety or effectiveness of BLUJEPA were observed between patients 65 years of age and older and younger adult patients, but greater sensitivity of some older individuals cannot be ruled out.
BLUJEPA is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function [see Warnings and Precautions (5.1), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
Uncomplicated Urogenital Gonorrhea
Trial 3 did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects.
8.6 Renal Impairment
For Patients with uUTI or Uncomplicated Urogenital Gonorrhea
No dosage adjustment is required in patients with mild renal impairment (eGFR 60 to 89 mL/min) or moderate renal impairment (eGFR 30 to 59 mL/min).
Avoid use of BLUJEPA in patients with severe renal impairment or kidney failure (eGFR <30 mL/min), including those receiving dialysis, due to increased exposure to gepotidacin and the risk of QTc prolongation [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
Additional Recommendations for Patients with Uncomplicated Urogenital Gonorrhea
For patients with uncomplicated urogenital gonorrhea and moderate renal impairment (eGFR 30 to 59 mL/min), avoid use of BLUJEPA when additional risk factors for increased gepotidacin exposure are present [see Warnings and Precautions (5.1)].
8.7 Hepatic Impairment
For Patients with uUTI or Uncomplicated Urogenital Gonorrhea
No dosage adjustment is required in patients with mild or moderate hepatic impairment (Child‑Pugh Class A/B).
Avoid use of BLUJEPA in patients with severe hepatic impairment (Child‑Pugh Class C) due to increased exposure to gepotidacin and the risk of QTc prolongation [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
Additional Recommendations for Patients with Uncomplicated Urogenital Gonorrhea
For patients with uncomplicated urogenital gonorrhea and moderate hepatic impairment (Child-Pugh Class B), avoid use of BLUJEPA when additional risk factors for increased gepotidacin exposure are present [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
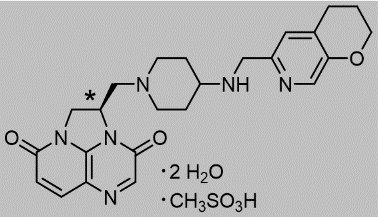
BLUJEPA tablets contain gepotidacin mesylate, a triazaacenaphthylene antibacterial that inhibits bacterial DNA gyrase and topoisomerase IV. The chemical name is (R)-2-((4-(((3,4-dihydro-2H-pyrano[2,3-c]pyridin-6-yl)methyl)amino)piperidin-1-yl)methyl)-1,2-dihydro-3H,8H-2a,5,8a-triazaacenaphthylene-3,8-dione methanesulfonate dihydrate. The molecular formula is C24H28N6O3●CH4O3S●2H2O and its molecular mass is 580.66. The structural formula is shown below.
*stereogenic center
Each BLUJEPA oral tablet contains gepotidacin 750 mg (equivalent to 910.7 mg of gepotidacin mesylate [anhydrous]). Inactive ingredients include colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
The 24-hour free‑drug AUC to minimum inhibitory concentration (MIC) ratio has been shown in animal infection and in vitro pharmacokinetic ‑pharmacodynamic (PK‑PD) models to be the PK‑PD index predictive of gepotidacin antibacterial efficacy.
Cardiac Electrophysiology
The effect of gepotidacin on the QTc interval was evaluated in a randomized, active (moxifloxacin 400 mg) and placebo‑controlled, double‑blind cross‑over trial in healthy subjects who received single intravenous (IV) infusions of gepotidacin over 2 hours. A dose- and concentration‑dependent QTc prolongation effect of gepotidacin was observed. The mean placebo‑corrected change from baseline heart rate values around Tmax were approximately 6 bpm at 1,000 mg IV (not an approved dosing regimen and route of administration) and approximately 10 bpm at 1,800 mg IV (not an approved dosing regimen and route of administration). The mean placebo‑corrected change from baseline QTcF values around Tmax were 12 msec at 1,000 mg IV and 22 msec at 1,800 mg IV [see Warnings and Precautions (5.1)]. The Cmax of gepotidacin following a single 1,000 mg IV dose (not an approved dosing regimen and route of administration) is approximately 1.2 times that of the Cmax at steady state for the 1,500 mg oral dose twice daily. The Cmax of gepotidacin following a single 1,800 mg IV dose (not an approved dosing regimen and route of administration) is approximately 1.2 times that of the Cmax after the second 3,000 mg oral dose given 12 hours after the first oral dose.
12.3 Pharmacokinetics
Pharmacokinetic Parameters
The pharmacokinetic properties of gepotidacin are summarized in Table 4 as mean (standard deviation [SD]) unless otherwise specified.
Table 4. Pharmacokinetic Parameters of Gepotidacin a Pharmacokinetic parameters are presented at steady state in patients with uUTI and eGFR greater than or equal to 90 mL/min after oral administration of BLUJEPA 1,500 mg every 12 hours over 5 days, and in simulated results in adults with eGFR greater than or equal to 90 mL/min after oral administration of 2 doses of BLUJEPA (3,000 mg) taken 12 hours apart. b AUC0-12 at steady state in patients with uUTI; simulated AUC0-24 on day 1 in simulated results with uncomplicated urogenital gonorrhea. c Studies evaluating the effect on food were performed with standard and moderate fat meal. Clinical studies were not performed with a high fat meal (1,000 calories, 50% fat). Dosing Regimen
1,500 mg Every 12 Hours over 5 Days
2 Doses of 3,000 mg 12 Hours Apart
Exposure
Cmax (mcg/mL)a
6.3 (1.0)
11 (2.7)
AUC (mcg*hour/mL)a,b
22.8 (4.8)
75.9 (25.9)
Dose Proportionality
Approximately dose proportional from 1,500 to 3,000 mg
Accumulation
40% and steady state was achieved by day 3
Absorption
Absolute Bioavailability
~45%
Tmax (hours)
~2.0
Effect of food (moderate fat meal)c
No clinically significant effect on PK
Distribution
Vss (L)a
172.9 (42.5)
188.0 (63.7)
Plasma Protein Binding
~25 to ~41%
Elimination
Terminal Half-life (hours)a
9.3 (1.3)
9.4 (2.3)
Total Clearance (L/hour)a
33.4 (6.7)
34.8 (8.7)
Metabolism
Primary Pathway
Oxidative metabolism mediated by CYP3A4, producing several circulating metabolites
Major Metabolite (%)
M4 which is ~11% of circulating drug‑related materials
Excretion
Feces
~52% (30% unchanged drug)
Urine
~31% (20% unchanged drug; major route of elimination for absorbed gepotidacin)
Specific Populations
Modelling and simulation analyses of gepotidacin showed that age, sex, and race have no clinically relevant effect on gepotidacin exposure. Body weight alone does not result in clinically significant effects on gepotidacin exposure [see Use in Specific Populations (8.4), (8.5)].
Patients with Renal Impairment: The pharmacokinetics of gepotidacin were evaluated in subjects with moderate renal impairment (eGFR 30 to 59 mL/min) and in subjects with severe renal impairment/end stage renal disease (ESRD) on intermittent hemodialysis and not on intermittent hemodialysis (eGFR <30 mL/min). Gepotidacin plasma Cmax and AUC in subjects with moderate renal impairment were 1.2-fold and 1.5-fold higher than matched healthy controls, respectively. Gepotidacin plasma Cmax and AUC in severe renal impairment/ESRD not on intermittent hemodialysis were 1.7-fold and 2.1‑fold higher than matched healthy controls, respectively. Gepotidacin plasma Cmax and AUC in ESRD subjects requiring intermittent hemodialysis were 2.3‑fold and 2.5-fold higher before intermittent hemodialysis than healthy matching subjects, respectively, and were 6.2‑fold and 4.2‑fold higher after intermittent hemodialysis than matched healthy controls, respectively [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment: Mild hepatic impairment did not have a clinically relevant effect on gepotidacin pharmacokinetics. Moderate hepatic impairment resulted in an approximately 1.2‑fold increase in gepotidacin plasma Cmax and AUC compared with normal hepatic function. In subjects with severe hepatic impairment compared with subjects with normal hepatic function, gepotidacin plasma exposure parameters (Cmax and AUC) were increased by approximately 1.9‑fold and 1.7‑fold, respectively [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Clinical Drug Interaction Studies and Model Informed Approaches:
Effect of CYP3A4 Strong Inhibitors on the Pharmacokinetics of Gepotidacin: Concomitant administration of a strong inhibitor of CYP3A4 (itraconazole; 200 mg per day for 3 days), and a single 1,500 mg dose of BLUJEPA resulted in an increase in the maximum concentration (Cmax) of gepotidacin of approximately 1.4‑fold and area under the curve (AUC) of approximately 1.5‑fold [see Drug Interactions (7.1)].
Effect of CYP3A4 Strong Inducers on the Pharmacokinetics of Gepotidacin: Concomitant administration of BLUJEPA (single 1,500 mg dose) with a strong CYP3A4 inducer (rifampin; 600 mg once daily for 7 days) resulted in a decrease of 52% in gepotidacin plasma AUC(0-∞) [see Drug Interactions (7.1)].
Effect of BLUJEPA on Pharmacokinetics of Other Drugs: Concomitant administration of a single 0.5 mg dose of digoxin with two 3,000 mg doses of BLUJEPA (an in vitro P‑glycoprotein inhibitor), given 12 hours apart (not an approved dosage of BLUJEPA), resulted in a 1.5‑fold increase in the digoxin Cmax (at 3 hours post dose), a 1.1‑fold increase in the digoxin AUC(0-∞), and a delayed digoxin Tmax [see Drug Interactions (7.2)].
Concomitant administration of midazolam (2 mg single dose) with BLUJEPA (2 doses of 3,000 mg, given 12 hours apart; not an approved dosage of BLUJEPA) resulted in a 1.9‑fold increase in midazolam AUC(0-∞) [see Drug Interactions (7.2)].
Effect of Moderate CYP3A Inhibitors on the Pharmacokinetics of Gepotidacin: Concomitant administration of a moderate CYP3A inhibitor (fluconazole) and single 1,500 mg dose of BLUJEPA is predicted to increase gepotidacin Cmax by 1.3‑fold and AUC by 1.5‑fold [see Drug Interactions (7.1)].
Effect of Moderate CYP3A Inducers on the Pharmacokinetics of Gepotidacin: Concomitant administration of a moderate CYP3A inducer (efavirenz) and single 1,500 mg dose of BLUJEPA is predicted to decrease AUC and Cmax by 49% and 34%, respectively [see Drug Interactions (7.1)].
In Vitro Drug Interaction Studies: In vitro, gepotidacin was not an inducer of CYP1A2, 2B6 or 3A4. In vitro, gepotidacin is not a substrate of any of the hepatic organic anion transporting polypeptides (OATPs) 1B1, 1B3, and 2B1, organic anion transporters (OATs) OAT1, OAT2 and OAT3, organic cation transporters (OCTs) OCT2 and OCT3.
In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically: In vitro, gepotidacin inhibited multidrug and toxin extrusion (MATEs) MATE1 (IC50=16.6 µM), and MATE2‑K (IC50=6.9 μM). In vitro, gepotidacin is a substrate of breast cancer resistance protein (max flux rate ratio of 11.0).
12.4 Microbiology
Mechanism of Action
BLUJEPA is a triazaacenaphthylene antibacterial that inhibits Type II topoisomerases including bacterial topoisomerase II (DNA gyrase) and topoisomerase IV, thereby inhibiting DNA replication.
Gepotidacin has bactericidal activity against pathogens as determined by time‑kill studies. In vitro studies demonstrated a gepotidacin post‑antibiotic effect ranging from 1.8 to 2.2 hours for E. coli, 1 to >6.6 hours for K. pneumoniae, 1.4 to 3 hours for P. mirabilis, 1 to 2.6 hours for C. freundii, 2.7 to 4.3 hours for S. saprophyticus, 1.2 to 2.7 hours for E. faecalis, and 0.7 to 1 hour for N. gonorrhoeae at 5 times the MIC.
Resistance
Although no clear mechanisms of resistance have been identified for gepotidacin, potential mechanisms that may impact gepotidacin activity are gepotidacin‑specific alterations of DNA gyrase (gyrA, gyrB) and/or topoisomerase IV (parC, parE) gene targets, plasmid‑mediated quinolone resistance genes (especially qnr), and efflux. The following amino acids may be important for gepotidacin activity GyrA P35, V44, D82, A175, GyrB D426, P445 and ParC D79 (based on E. coli numbering) as shown through studies with isogenic mutants in E. coli and K. pneumoniae. The amino acids GyrA D90, A92 and ParC D86 (based on N. gonorrhoeae numbering) as shown through studies with isogenic mutants in N. gonorrhoeae may also be important. A single target‑specific mutation may not significantly impact gepotidacin activity. In studies of certain amino acid substitutions in GyrA and ParC of E.coli and N. gonorrhoeae, a direct relationship between gepotidacin and fluoroquinolone susceptibility was not established. However, in a study of isogenic N. gonorrhoeae strains with mutations in GyrA S91F, A92T, D95G, and ParC D86N, resistance to both gepotidacin and ciprofloxacin was observed. The clinical significance of these findings is unknown. Gepotidacin activity against E. coli, K. pneumoniae, and N. gonorrhoeae is unrelated to beta‑lactam resistance mechanisms.
The frequency of resistance development to gepotidacin due to spontaneous mutations in the gram‑negative pathogens and gram‑positive uropathogens tested in vitro at 10 times MIC ranged from 10-9 to 10-10.
Target‑specific cross‑resistance with other classes of antibacterial drugs has not been identified; therefore, isolates resistant to other drugs may be susceptible to gepotidacin. However, isolates of Enterobacterales with ≥4‑fold increases in gepotidacin MIC have been identified in vitro and in clinical studies. Using an unapproved single dose of ≤3,000 mg of gepotidacin, three microbiological failures were reported among patients from the Phase 2 clinical study with N. gonorrhoeae isolates with a baseline MIC of 1 mcg/mL. Two of these isolates at the end of treatment, had mutations in GyrA and ParC known to be important for gepotidacin binding to the target (ParC D86N, GyrA A92T), and resistance to gepotidacin (MIC increased ≥32‑fold). Using 3,000 mg of gepotidacin followed by a second 3,000 mg dose taken approximately 12 hours later, no N. gonorrhoeae isolates with gepotidacin MIC values >2 mcg/mL were observed, including for pre‑treatment isolates with a ParC D86N mutation.
During clinical studies, gepotidacin demonstrated activity against some isolates of the following multilocus sequence typing (MLST) for E. coli: ST10, ST131, ST1193, ST69, ST95 and ST73. In vitro activity was also demonstrated against the following N. gonorrhoeae MLSTs: ST11422, ST11706, ST1580, ST1583, ST7363, ST7822 and ST9362.
Interaction with Other Antimicrobials
In in vitro studies, no antagonism against Enterobacterales or gram‑positive isolates was observed for gepotidacin in combination with multiple antibacterial drugs, including fluoroquinolones, sulfonamides, cephalosporins, macrolides, tetracyclines, aminoglycosides, glycopeptides, carbapenems, nitrofurans, monobactams, and oxazolidinones, or for N. gonorrhoeae for gepotidacin in combination with fluoroquinolones, cephalosporins, macrolides, tetracyclines, aminoglycosides, pleuromutilins, and aminocyclitols.
Antimicrobial Activity
Gepotidacin has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)]:
Uncomplicated UTI:
Aerobic bacteria
Gram‑positive bacteria
Enterococcus faecalis
Staphylococcus saprophyticus
Gram‑negative bacteria
Citrobacter freundii complex
Escherichia coli
Klebsiella pneumoniae
Uncomplicated Urogenital Gonorrhea:
Aerobic bacteria
Gram‑negative bacteria
Neisseria gonorrhoeae
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for gepotidacin against isolates of similar genus or organism group. However, the efficacy of gepotidacin in treating clinical infections caused by these bacteria has not been established in adequate and well‑controlled clinical trials.
Aerobic bacteria
Gram‑negative bacteria
Citrobacter koseri
Klebsiella aerogenes
Klebsiella oxytoca/Raoltella ornithinolytica
Morganella morganii
Proteus mirabilis
Providencia rettgeri
Susceptibility Test Methods
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long term carcinogenicity studies have not been conducted with gepotidacin.
Mutagenesis
Gepotidacin was positive in an in vitro micronucleus test in human peripheral blood lymphocytes and in an L5178Y mouse lymphoma assay, consistent with the known in vitro clastogenic effects of topoisomerase inhibitors in in vitro mammalian cell assays. Gepotidacin was negative in an in vivo micronucleus test or Comet assay in rat. Based on an overall weight of evidence, gepotidacin is unlikely to be genotoxic.
Impairment of Fertility
In animal studies with gepotidacin, there were no adverse effects on fertility in male and female rats treated for approximately 3 weeks at approximately 2 times the exposure at the MRHD (AUC extrapolated from rats orally administered the same dose for up to 4 weeks). There were no effects on spermatogenesis in rats and dogs at approximately 2 and 3 times the exposure at MRHD, respectively, when treated for up to 13 weeks.
-
14 CLINICAL STUDIES
14.1 Uncomplicated Urinary Tract Infections
A total of 3,136 female patients with uncomplicated urinary tract infections (uUTI) were randomized in 2 multicenter, parallel‑group, double‑blind, double‑dummy, non‑inferiority (NI) trials (Trial 1 [NCT04020341] and Trial 2 [NCT04187144]). Both trials compared BLUJEPA 1,500 mg (administered orally twice daily with food for 5 days) to nitrofurantoin 100 mg (administered orally twice daily for 5 days).
Patients entered the trials with at least 2 symptoms consistent with uUTI (dysuria, frequency, urgency, or lower abdominal pain) and with evidence of urinary nitrite or pyuria. Patients with any medical condition or presentation suggestive of a complicated UTI, or an upper UTI (e.g., pyelonephritis, urosepsis) were excluded.
Efficacy was assessed as a composite of clinical cure and microbiological response at the Test‑of‑Cure (TOC) Visit (study day 10 to 13) in the microbiological ITT nitrofurantoin‑susceptible (micro‑ITTS) population, which included all patients who received at least 1 dose of study medication, had at least 1 baseline qualifying uropathogen (≥105 colony‑forming units [CFU]/mL), and excluded patients with organisms not susceptible to nitrofurantoin. Clinical cure was defined as resolution of all signs and symptoms of acute cystitis present at baseline and no new signs and symptoms without the patient receiving other systemic antimicrobials.
Microbiological response was defined as having all qualifying uropathogens found at baseline at ≥105 CFU/mL reduced to <103 CFU/mL without the patient receiving other systemic antimicrobials.
Both trials demonstrated non‑inferiority of BLUJEPA to nitrofurantoin for composite response (Table 5).
In Trial 1, the micro‑ITTS population consisted of 634 female patients with uUTI (n = 336 BLUJEPA; n = 298 nitrofurantoin). The median age of patients was 54 years, 57% were >50 years of age, 84% were White, 40% had a history of recurrent infection. The U.S. enrolled the greatest percentage of patients (39%). Patient demographic and baseline characteristics were generally balanced between treatment groups [see Adverse Reactions (6.1)].
In Trial 2, the micro‑ITTS population consisted of 567 female patients with uUTI (n = 292 BLUJEPA; n = 275 nitrofurantoin). The median age of patients was 51 years, 52% were >50 years of age, 85% were White, 41% had history of recurrent infection. The majority of patients (67%) were enrolled from the United States. Patient demographic and baseline characteristics were generally balanced between treatment groups [see Adverse Reactions (6.1)].
Table 5 summarizes the composite response, clinical cure, and microbiological response rates at the TOC visit for Trials 1 and 2 in the micro‑ITTS population.
Table 5. Composite Response, Clinical Cure, and Microbiological Response Rates at the Test-of-Cure Visit (Micro-ITTS Population) micro-ITTS = microbiological Intent to Treat nitrofurantoin-susceptible; CI = confidence interval. a BLUJEPA was non-inferior to nitrofurantoin in both studies. The determination of Trial 1 non‑inferiority was based on a planned interim analysis of 607 subjects in micro-ITTS population. The determination of Trial 2 non‑inferiority was based on a planned interim analysis of 541 subjects in micro-ITTS population. b Treatment difference (BLUJEPA – nitrofurantoin) calculated using Miettinen and Nurminen Summary Score method adjusting for age group and recurrent/non-recurrent infection status combinations. Study Endpoint
BLUJEPA
n/N (%)
Nitrofurantoin
n/N (%)
Treatment Difference
(95% CI)b
Trial 1
Composite responsea
174/336 (51.8)
140/298 (47.0)
5.3 (-2.4, 13.0)
Clinical cure
224/336 (66.7)
196/298 (65.8)
1.5 (-5.8, 8.8)
Microbiological response
244/336 (72.6)
199/298 (66.8)
6.0 (-1.2, 13.1)
Trial 2
Composite responsea
172/292 (58.9)
121/275 (44.0)
14.4 (6.4, 22.4)
Clinical cure
199/292 (68.2)
175/275 (63.6)
4.3 (-3.4, 12.0)
Microbiological response
213/292 (72.9)
158/275 (57.5)
15.5 (7.9, 23.1)
Table 6 summarizes the composite response rates at the TOC Visit for the most common baseline uropathogens across both trials in the micro-ITTS population.
Table 6. Composite Response Rates at the Test-of-Cure Visit by Baseline Uropathogen (Trial 1 and Trial 2 Pooled; Micro-ITTS Population)a micro‑ITTS = microbiological Intent to Treat nitrofurantoin-susceptible a A patient is counted once under a uropathogen category if multiple qualifying uropathogens within that category are isolated at baseline for the patient. b Patients may have been infected with 1 to 2 uropathogens at baseline. Pathogenb
BLUJEPAa
n/N (%)
Nitrofurantoina
n/N (%)
Escherichia coli
312/566 (55.1)
234/520 (45.0)
Klebsiella pneumoniae
6/14 (42.9)
6/16 (37.5)
Staphylococcus saprophyticus
9/15 (60.0)
11/14 (78.6)
Enterococcus faecalis
8/14 (57.1)
2/7 (28.6)
Citrobacter freundii complex
8/12 (66.7)
2/5 (40.0)
14.2 Uncomplicated Urogenital Gonorrhea
A total of 628 patients with suspected uncomplicated urogenital gonorrhea due to Neisseria gonorrhoeae were randomized in an open‑label, active‑controlled, multicenter, multinational trial (Trial 3; NCT04010539). Patients were randomized 1:1 to receive either BLUJEPA (3,000 mg taken orally followed by a second dose of 3,000 mg approximately 12 hours later), or a combination of a single intramuscular 500 mg dose of ceftriaxone and single 1 g oral dose of azithromycin.
Patients were eligible for enrollment if they were ≥12 years old and >45 kg. Patients with confirmed or suspected complicated or disseminated gonorrhea were excluded from the study. The microbiological intent‑to‑treat (micro‑ITT) population, which included patients who had urogenital N. gonorrhoeae isolated at baseline and who were not infected with a strain that was nonsusceptible to ceftriaxone at baseline, consisted of 406 patients (202 for BLUJEPA and 204 for ceftriaxone and azithromycin). The demographic and baseline characteristics in the micro‑ITT population were comparable between treatment groups. In total, 92% were male; 74% White, 15% Black, 6% Asian, 17% Hispanic or Latino; the mean age was 33 years (range: 17 to 64); the mean weight was 76 kg (range: 48 to 120 kg). The primary efficacy endpoint was microbiological success as determined by confirmed bacterial eradication of N. gonorrhoeae at the urogenital body site at the test of cure (TOC) visit (Day 4 to 8) without receipt of other systemic antimicrobials and was assessed in the micro‑ITT population.
Table 7 shows the primary endpoint of the microbiological success rates at the urogenital site at TOC in the micro‑ITT population. Trial 3 demonstrated non‑inferiority of BLUJEPA to the combination of ceftriaxone and azithromycin.
Table 7. Microbiological Success Rates at the Urogenital Site at TOC, Micro-ITT Population (Trial 3) TOC = Test of Cure; micro‑ITT = microbiological Intent to Treat; CI=confidence interval; n = number of patients in subcategory; N = number of patients in the specified population a Calculated using Miettinen and Nurminen Summary Score method adjusting for sex and sexual orientation (female, men who have sex with men, or men who have sex with women) for the BLUJEPA – (ceftriaxone and azithromycin) treatment difference. b Unable to determine outcomes were due to missing data, culture processed beyond 24 hours, TOC out of window, or use of another systemic antimicrobial prior to the TOC visit BLUJEPA
n/N (%)
Ceftriaxone and Azithromycin
n/N (%)
Treatment Difference
(95% CI)a
Microbiological success
187/202 (92.6)
186/204 (91.2)
-0.1 (-5.6, 5.5)
Microbiological failure
15/202 (7.4)
18/204 (8.8)
Bacterial persistence by culture
0
0
Unable to determineb
15/202 (7.4)
18/204 (8.8)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
BLUJEPA tablets are supplied as yellow, film-coated, capsule-shaped tablets debossed with “GS GU3” on one side and plain on the other side, containing 750 mg of gepotidacin.
Bottle of 8 tablets (NDC: 0173-0922-38).
Bottle of 20 tablets (NDC: 0173-0922-45).
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). [See USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Important Administration Instructions
Counsel patients to take BLUJEPA after a meal to reduce the possibility of gastrointestinal intolerance [see Dosage and Administration (2.3)].
Prolongation of the QTc Interval
Counsel patients to inform their healthcare provider of any personal or family history of QTc prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia, or if they are taking any antiarrhythmic agents. Advise patients to notify their healthcare providers if they have any symptoms of prolongation of the QTc interval, including prolonged heart palpitations or a loss of consciousness [see Warnings and Precautions (5.1)].
Acetylcholinesterase Inhibition
Counsel patients that BLUJEPA can cause dysarthria and other symptoms such as syncope, presyncope, muscle spasms, diarrhea, nausea, vomiting, abdominal pain, hypersalivation, and hyperhidrosis. Advise patients to inform their healthcare provider if they experience these symptoms or if they have an underlying medical condition that may be exacerbated by acetylcholinesterase inhibition or are planning to receive anesthesia where they may receive neuromuscular blocking agents, or if they are receiving other acetylcholinesterase inhibitors, or systemic anticholinergic medications [see Warnings and Precautions (5.2), Drug Interactions (7.3)].
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions, including anaphylaxis, could occur and require immediate treatment. Advise patients to inform their healthcare provider about any previous hypersensitivity reactions to BLUJEPA [see Warnings and Precautions (5.3)].
Diarrhea
Counsel patients that diarrhea is a common problem caused by antibacterials, including BLUJEPA. Most reported cases of diarrhea with BLUJEPA were mild to moderate in severity. Diarrhea may occur early in treatment and usually ends when treatment with the antibacterial is discontinued. In most cases, the diarrhea resolved without treatment [see Warnings and Precautions (5.2), Adverse Reactions (6.1)].
Sometimes potentially serious diarrhea with frequent watery or bloody diarrhea may occur and may be a sign of a more serious intestinal infection. If severe watery or bloody diarrhea develops, patients should contact their healthcare provider [see Warnings and Precautions (5.4)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs, including BLUJEPA, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When BLUJEPA is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by BLUJEPA or other antibacterial drugs in the future [see Warnings and Precautions (5.5)].
Drug Interactions
Advise patients of the potential interactions other medications may have with BLUJEPA or the effect BLUJEPA may have on other medications, as these may result in decreased effectiveness or increased toxicities of either BLUJEPA or the other medications. Patients should alert their healthcare provider if they are currently taking any medications, including herbal nutritional supplements, or are prescribed new medications during treatment with BLUJEPA [see Warnings and Precautions (5.1), Drug Interactions (7), Clinical Pharmacology (12.3)].
Pregnancy
Advise patients who are exposed to BLUJEPA during pregnancy to contact GlaxoSmithKline at 1‑888‑825‑5249 [see Use in Specific Populations (8.1)].
Manufactured for:
GlaxoSmithKline
Durham, NC 27701
Trademarks are owned by or licensed to the GSK group of companies.
©2026 GSK group of companies or its licensor.
BLJ:3PI
-
MEDICATION GUIDE
MEDICATION GUIDE
BLUJEPA (blu – JEP – ah)
(gepotidacin)
tablets, for oral use
What is the most important information I should know about BLUJEPA?
BLUJEPA, an antibiotic, can cause serious side effects, including:
-
changes in electrical activity of your heart called QTc prolongation. BLUJEPA can cause QTc prolongation (also called prolonged QTc interval) that can lead to serious heart rhythm problems, including Torsade de Pointes. There may be changes in your heartbeat (a fast or irregular heartbeat).
Before starting BLUJEPA, tell your healthcare provider if you:- o or anyone in your family has ever had a heart rhythm problem (problem with heart rate or heartbeat) or QTc prolongation.
- o are taking any medicines to treat heart rhythm problems. Using these medicines with BLUJEPA may cause serious side effects. If you are not sure, ask your healthcare provider.
- o are taking medicines called moderate or strong CYP3A4 inhibitors. Using these medicines with BLUJEPA may cause serious side effects. If you are not sure, ask your healthcare provider.
Tell your healthcare provider right away if you have any of the following symptoms:
- ○fast, pounding, or uneven heartbeat
- ○feeling dizzy
- ○you lose consciousness (pass out)
- ○feeling lightheaded
- ○feeling faint
- an increase of a certain chemical in your nervous system (cholinergic effects). BLUJEPA can cause an increase of a certain chemical in your nervous system, which may cause the following symptoms:
- ○diarrhea
- ○vomiting
- ○fainting
- ○trouble saying words clearly
- ○muscle spasms
- ○nausea
- ○more saliva in the mouth
- ○more sweating
- ○stomach pain
Call your healthcare provider right away if you have trouble saying words clearly, have shortness of breath, or if you faint. These symptoms may be cholinergic effects of BLUJEPA.
The increased chemical in your nervous system can also be connected to other symptoms. Call your healthcare provider right away if you have any of these additional symptoms.
- ○irregular heartbeat (heart block)
- ○slow heartbeat (bradycardia)
- ○seizures
- ○fainting
- ○chest tightness causing difficulty breathing
- allergic reactions. Allergic reactions can happen in people who take BLUJEPA including a severe allergic reaction called anaphylaxis. Stop taking BLUJEPA and get medical help right away if you have any symptoms of a severe allergic reaction, including:
- ○hives
- ○feeling faint or dizzy
- ○rash
- ○swelling of your lips, tongue, or throat
- diarrhea. Diarrhea is a common side effect caused by antibiotics including BLUJEPA. The diarrhea may happen at the beginning of your treatment with BLUJEPA and usually stops after the antibiotic is stopped. In most cases the diarrhea will go away without treatment. In some cases, diarrhea may be caused by Clostridioides difficile infection (CDI). CDI is a severe infection of the intestines (bowels) that can happen up to 2 months after finishing treatment with antibiotic medicines, including BLUJEPA. CDI can be life-threatening and can lead to death. Do not take medicines to treat diarrhea without checking first with your healthcare provider. Call your healthcare provider right away if you have the following symptoms:
- ○stomach cramps
- ○fever
- ○watery diarrhea
- ○diarrhea that does not go away
- ○bloody stools
What is BLUJEPA?
BLUJEPA is an antibiotic used to treat:
- Women and girls 12 years of age and older weighing at least 88 pounds who have an infection of the bladder (known as an uncomplicated urinary tract infection [uUTI]) caused by certain types of bacteria.
- Adults and children 12 years of age and older weighing at least 99 pounds who have very few or no other medicine choices to treat a sexually transmitted disease called uncomplicated urogenital gonorrhea (gonorrhea) caused by a certain type of bacteria.
- It is not known if BLUJEPA is safe and effective for the treatment of uUTI in children under 12 years of age or weighing less than 88 pounds.
- It is not known if BLUJEPA is safe and effective for the treatment of gonorrhea in children under 12 years of age or weighing less than 99 pounds.
Do not take BLUJEPA if:
you have had a severe allergic reaction to BLUJEPA. See the end of this Medication Guide for a complete list of ingredients in BLUJEPA.
Before taking BLUJEPA, tell your healthcare provider about all of your medical conditions, including if you:
- have heart rhythm problems. See “What is the most important information I should know about BLUJEPA?”
- have a slow heartbeat (bradycardia).
- have recently had a heart attack.
- have low potassium in your blood (hypokalemia).
- are planning to have a medical or surgical procedure that puts you to sleep (general anesthesia) that will use a type of medicine given through your veins (I.V.) that relaxes your muscles.
- have liver problems.
- have kidney problems, kidney failure, or are on dialysis.
- are pregnant or plan to become pregnant. It is not known if BLUJEPA will harm your unborn baby.
Tell your healthcare provider if you become pregnant or think you are pregnant during your treatment with BLUJEPA.
- Pregnancy Exposure Registry: There is a pregnancy exposure registry for women who are exposed to BLUJEPA during pregnancy. The purpose of this registry is to check the health of you and your baby. If you are pregnant or become pregnant during your treatment with BLUJEPA, talk to your healthcare provider about registering with GlaxoSmithKline. You can register by calling GlaxoSmithKline at 1‑888‑825‑5249.
- are breastfeeding or plan to breastfeed. It is not known if BLUJEPA passes into breast milk. Talk to your healthcare provider about the best way to feed your baby during your treatment with BLUJEPA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. BLUJEPA and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take medicines to treat heart rhythm problems.
Ask your healthcare provider or pharmacist, if you are not sure if you are taking any of these medicines. Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take BLUJEPA?
- Take BLUJEPA exactly as your healthcare provider tells you to take it. Check with your healthcare provider if you are not sure.
- The dose of BLUJEPA for the treatment of uUTI, is 2 tablets by mouth, 2 times each day (about 12 hours apart), for 5 days.
- The dose of BLUJEPA for the treatment of gonorrhea is 4 tablets taken by mouth followed by a second dose of 4 tablets about 12 hours later.
- Take BLUJEPA after a meal to decrease the chance of stomach upset.
- For the treatment of uUTI:
- ○If you miss a dose, take it as soon as possible, then continue your treatment as before. Do not take 2 doses to make up for a missed dose.
- For the treatment of gonorrhea:
- ○If you miss a dose, take it as soon as possible.
- If you take too much BLUJEPA, call the Poison Help line at 1‑800‑222‑1222 or go to the nearest hospital emergency room right away.
- Take the full course of treatment with BLUJEPA. Do not stop taking BLUJEPA during your treatment unless your healthcare provider tells you to, even if you are feeling better. If you do not complete your full course of treatment, the infection may come back.
What are the possible side effects of BLUJEPA?
BLUJEPA can cause serious side effects including:
- See “What is the most important information I should know about BLUJEPA?”
The most common side effects of BLUJEPA in people with uUTI include:
- ○diarrhea
- ○nausea
- ○stomach pain
- ○gas
- ○headache
- ○soft stools
- ○dizziness
- ○vomiting
- ○yeast infections
The most common side effects of BLUJEPA in people with gonorrhea include:
- ○diarrhea
- ○nausea
- ○stomach pain
- ○vomiting
- ○gas
- ○dizziness
- ○soft stools
- ○headache
- ○feeling tired
- ○excessive sweating
These are not all the possible side effects of BLUJEPA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store BLUJEPA?
- Store BLUJEPA tablets at room temperature between 68°F to 77°F (20°C to 25°C).
Keep BLUJEPA and all medicines out of the reach of children.
General information about the safe and effective use of BLUJEPA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BLUJEPA for a condition for which it was not prescribed. Do not give BLUJEPA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about BLUJEPA that is written for health professionals.
What are the ingredients in BLUJEPA?
Active ingredient: gepotidacin mesylate
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide and yellow iron oxide.
Manufactured for:
For more information, call 1‑888‑825‑5249 or go to www.gsk.com.
Trademarks are owned by or licensed to the GSK group of companies.
GlaxoSmithKline, Durham, NC 27701
©2025 GSK group of companies or its licensor.
BLJ:2MG
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 12/2025
-
changes in electrical activity of your heart called QTc prolongation. BLUJEPA can cause QTc prolongation (also called prolonged QTc interval) that can lead to serious heart rhythm problems, including Torsade de Pointes. There may be changes in your heartbeat (a fast or irregular heartbeat).
-
PRINCIPAL DISPLAY PANEL
PRINCICPAL DISPLAY PANEL
NDC: 0173-0922-45
BLUJEPA
(gepotidacin)
tablets
750 mg
Rx only
Dispense the accompanying Medication Guide to each patient
20 tablets
GSK
Each tablet contains gepotidacin 750 mg (equivalent to 910.7 mg of gepotidacin mesylate [anhydrous]).
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature].
Keep out of reach of children.
Do not accept if safety seal under cap is missing or broken.
Recommended Dosage: see Prescribing Information.
Trademarks owned or licensed by GSK.
Mft. For: GSK, Durham, NC 27701
Scan for product information or visit epi-pla.org
Made in Belgium
©2025 GSK or licensor. Rev. 03/25
2000017942
-
INGREDIENTS AND APPEARANCE
BLUJEPA
gepotidacin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0173-0922 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GEPOTIDACIN MESYLATE (UNII: 5P7X0H2O6B) (GEPOTIDACIN - UNII:DVF0PR037D) GEPOTIDACIN 750 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score no score Shape CAPSULE Size 21mm Flavor Imprint Code G5;GU3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0173-0922-45 20 in 1 BOTTLE; Type 0: Not a Combination Product 03/25/2025 2 NDC: 0173-0922-38 8 in 1 BOTTLE; Type 0: Not a Combination Product 12/11/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218230 03/25/2025 Labeler - GlaxoSmithKline LLC (167380711)
Trademark Results [Blujepa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BLUJEPA 98126258 not registered Live/Pending |
Glaxo Group Limited 2023-08-10 |
 BLUJEPA 97254029 not registered Live/Pending |
Glaxo Group Limited 2022-02-04 |
 BLUJEPA 86942163 5059477 Live/Registered |
Glaxo Group Limited 2016-03-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.