DetomiSed™(detomidine hydrochloride)
Detomised by
Drug Labeling and Warnings
Detomised by is a Animal medication manufactured, distributed, or labeled by Akorn, Grindeks JSC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DETOMISED- detomidine hydrochloride solution
Akorn
----------
DetomiSed™
(detomidine hydrochloride)
Sedative and Analgesic For Use in Horses Only
Sterile Solution
10 mg/mL
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION:
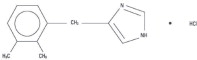
DetomiSed™ is a synthetic alpha-2 adrenoreceptor agonist with sedative and analgesic properties. The chemical name is 1H imidazole, 4-[(2,3-dimethylphenyl) methyl]-hydrochloride and the generic name is detomidine hydrochloride. It is a white, crystalline, water-soluble substance having a molecular weight of 222.7. The molecular formula is C12H14N2-HCl.
CHEMICAL STRUCTURE:
Each mL of DetomiSed™ contains 10.0 mg detomidine hydrochloride, 1.0 mg methyl paraben, 5.9 mg sodium chloride, and water for injection, q.s.
CLINICAL PHARMACOLOGY:
DetomiSed™, a non-narcotic sedative and analgesic, is a potent α2-adrenoreceptor agonist which produces sedation and superficial and visceral analgesia which is dose dependent in its depth and duration. Profound lethargy and a characteristic lowering of the head with reduced sensitivity to environmental stimuli (sounds, etc.) are seen with detomidine. A short period of incoordination is characteristically followed by immobility and a firm stance with front legs well spread. The analgesic effect is most readily seen as an increase in the pain threshold at the body surface. Sensitivity to touch is little affected and in some cases may actually be enhanced.
With detomidine administration, heart rate is markedly decreased, blood pressure is initially elevated, and then a steady decline to normal is seen. A transient change in the conductivity of the cardiac muscle may occur, as evidenced by partial atrioventricular (AV) and sinoauricular (SA) blocks. This change in the conductivity of the cardiac muscle may be prevented by IV administration of atropine at 0.02 mg/kg of body weight.
No effect on blood clotting time or other hematological parameters was encountered at dosages of 20 or 40 mcg/kg of body weight. Respiratory responses include an initial slowing of respiration within a few seconds to 1 to 2 minutes after administration, increasing to normal within 5 minutes. An initial decrease in tidal volume is followed by an increase.
INDICATIONS:
DetomiSed™ is indicated for use as a sedative and analgesic to facilitate minor surgical and diagnostic procedures in mature horses and yearlings. It has been used successfully for the following: to calm fractious horses, to provide relief from abdominal pain, to facilitate bronchoscopy, bronchoalveolar lavage, nasogastric intubation, nonreproductive rectal palpations, suturing of skin lacerations, and castrations. Additionally, an approved, local infiltration anesthetic is indicated for castration.
CONTRAINDICATIONS:
DetomiSed™ should not be used in horses with pre-existing AV or SA block, with severe coronary insufficiency, cerebrovascular disease, respiratory disease, or chronic renal failure. Intravenous potentiated sulfonamides should not be used in anesthetized or sedated horses as potentially fatal dysrhythmias may occur.
Information on the possible effects of detomidine hydrochloride in breeding horses is limited to uncontrolled clinical reports; therefore, this drug is not recommended for use in breeding animals.
WARNINGS:
 | Do not use in horses intended for human consumption. Not for human use. Keep out of reach of children. |  |
HUMAN SAFETY INFORMATION:
Care should be taken to assure that detomidine hydrochloride is not inadvertently ingested as safety studies have indicated that the drug is well absorbed when administered orally. Standard ocular irritation tests in rabbits using the proposed market formulation have shown detomidine hydrochloride to be nonirritating to eyes. Primary dermal irritation tests in guinea pigs using up to 5 times the proposed market concentration of detomidine hydrochloride on intact and abraded skin have demonstrated that the drug is nonirritating to skin and is apparently poorly absorbed dermally. However, in accordance with prudent clinical procedures, exposure of eyes or skin should be avoided and affected areas should be washed immediately if exposure does occur. As with all injectable drugs causing profound physiological effects, routine precautions should be employed by practitioners when handling and using loaded syringes to prevent accidental self-injection.
PRECAUTIONS:
Before administration, careful consideration should be given to administering DetomiSed™ to horses approaching or in endotoxic or traumatic shock, to horses with advanced liver or kidney disease, or to horses under stress from extreme heat, cold, fatigue, or high altitude. Protect treated horses from temperature extremes. Some horses, although apparently deeply sedated, may still respond to external stimuli. Routine safety measures should be employed to protect practitioners and handlers. Allowing the horse to stand quietly for 5 minutes before administration and for 10 to 15 minutes after injection may improve the response to DetomiSed™.
DetomiSed™ is a potent α2-agonist, and extreme caution should be exercised in its use with other sedative or analgesic drugs for they may produce additive effects.
When using any analgesic to help alleviate abdominal pain, a complete physical examination and diagnostic work-up are necessary to determine the etiology of the pain.
Food and water should be withheld until the sedative effect of DetomiSed™ has worn off.
ADVERSE REACTIONS:
Occasional reports of anaphylactic-like reactions have been received, including 1 or more of the following: urticaria, skin plaques, dyspnea, edema of the upper airways, trembling, recumbency, and death. The use of epinephrine should be avoided since epinephrine may potentiate the effects of α2-agonists. Reports of mild adverse reactions have resolved uneventfully without treatment. Severe adverse reactions should be treated symptomatically. As with all α2-agonists, the potential for isolated cases of hypersensitivity exist, including paradoxical response (excitation).
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet, contact Akorn Operating Company LLC at 1-800-932-5676 or www.akorn.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reoortanimalae
SIDE EFFECTS:
Horses treated with DetomiSed™ exhibit hypertension. Bradycardia routinely occurs 1 minute after injection. The relationship between hypertension and bradycardia is consistent with an adaptive baroreceptor response to the increased pressure and inconsistent with a primary drug-induced bradycardia. Piloerection, sweating, salivation, and slight muscle tremors are frequently seen after administration. Partial transient penis prolapse may be seen. Partial AV and SA blocks may occur with decreased heart and respiratory rates. Urination typically occurs during recovery at about 45 to 60 minutes posttreatment, depending on dosage. Incoordination or staggering is usually seen only during the first 3 to 5 minutes after injection, until animals have secured a firm footing.
Because of continued lowering of the head during sedation, mucus discharges from the nose and, occasionally, edema of the head and face may be seen. Holding the head in a slightly elevated position generally prevents these effects.
OVERDOSAGE:
Detomidine hydrochloride is tolerated in horses at up to 200 mcg/kg of body weight (10 times the low dosage and 5 times the high dosage). In safety studies in horses, detomidine hydrochloride at 400 mcg/kg of body weight administered daily for 3 consecutive days produced microscopic foci of myocardial necrosis in 1 of 8 horses.
DOSAGE AND ADMINISTRATION:
For Sedation: Administer DetomiSed™ IV or IM at the rates of 20 or 40 mcg detomidine hydrochloride per kg of body weight (0.2 or 0.4 mL of DetomiSed™ per 100 kg or 220 lb), depending on the depth and duration of sedation required. Onset of sedative effects should be reached within 2 to 4 minutes after IV administration and 3 to 5 minutes after IM administration. Twenty mcg/kg will provide 30 to 90 minutes of sedation and 40 mcg/kg will provide approximately 90 minutes to 2 hours of sedation.
For Analgesia: Administer DetomiSed™ IV at the rates of 20 or 40 mcg detomidine hydrochloride per kg of body weight (0.2 or 0.4 mL of DetomiSed™ per 100 kg or 220 lb), depending on the depth and duration of analgesia required. Twenty mcg/kg will usually begin to take effect in 2 to 4 minutes and provide 30 to 45 minutes of analgesia. The 40 mcg/kg dose will also begin to take effect in 2 to 4 minutes and provide 45 to 75 minutes of analgesia.
For Both Sedation and Analgesia: Administer DetomiSed™ IV at the rates of 20 or 40 mcg detomidine hydrochloride per kg of body weight (0.2 or 0.4 mL of DetomiSed™ per 100 kg or 220 lb), depending on the depth and duration of sedation and analgesia required.
Before and after injection, the animal should be allowed to rest quietly.
STORAGE:
Store at (20° to 25°C) in the absence of light; excursions permitted to 40°C.
Use contents within 28 days of first puncture.
HOW SUPPLIED:
DetomiSed™ is supplied in 5 mL and 20 mL multi-dose vials.
NDC: 59399-105-05 5 mL vial in package of one
NDC: 59399-105-20 20 mL vial in package of one
Approved by FDA under ANADA # 200-611
AKORN
Distributed by: Akorn Operating Company LLC
Gurnee, IL 60031
VDH00N Rev. 05/22
Principal Display Panel Text for Container Label:
NDC: 59399-105-05
DetomiSed™
(detomidine hydrochloride)
Sedative and Analgesic
For Use in Horses Only
Sterile Solution – 10 mg/mL
Caution: Federal law restricts this
drug to use by or on the order of a
licensed veterinarian.
Net Contents: 5 mL
Approved by FDA under ANDA # 200-611
Principal Display Panel Text for Carton Label:
NDC: 59399-105-05
DetomiSed™
(detomidine hydrochloride)
Sedative and Analgesic
For Use in Horses Only
Sterile Solution – 10 mg/mL
Caution: Federal law restricts
this drug to use by or on the
order of a licensed veterinarian.
Net Contents: 5 mL
Approved by FDA under
ANDA # 200-611
Akorn logo
| DETOMISED
detomidine hydrochloride solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Akorn (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696832 | MANUFACTURE, ANALYSIS, STERILIZE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696790 | LABEL, PACK | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Grindeks JSC | 644702888 | API MANUFACTURE | |
Trademark Results [Detomised]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DETOMISED 98605908 not registered Live/Pending |
Cronus Pharma LLC 2024-06-18 |
 DETOMISED 88677127 not registered Live/Pending |
Akorn Animal Health, Inc. 2019-11-01 |
 DETOMISED 88677127 not registered Live/Pending |
AKORN OPERATING COMPANY LLC 2019-11-01 |
 DETOMISED 86478401 not registered Dead/Abandoned |
Akorn Animal Health, Inc. 2014-12-11 |
 DETOMISED 85794314 not registered Dead/Abandoned |
Akorn Animal Health, Inc. 2012-12-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.