Carbamazepine by Aidarex Pharmaceuticals LLC CARBAMAZEPINE tablet
Carbamazepine by
Drug Labeling and Warnings
Carbamazepine by is a Prescription medication manufactured, distributed, or labeled by Aidarex Pharmaceuticals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use carisoprodol safely and effectively. See full prescribing information for carisoprodol tablets, USP.
Carisoprodol Tablets, USP for Oral use CIV
Initial U.S. Approval: 1959RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Recommended dose is 350 mg three times a day and at bedtime. (2)
DOSAGE FORMS AND STRENGTHS
Tablets: 350 mg (3)CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence > 2%) are drowsiness, dizziness, and headache (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2012
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Sedation
5.2 Drug Dependence, Withdrawal, and Abuse
5.3 Seizures
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CNS Depressants
7.2 CYP2C19 Inhibitors and Inducers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Pregnancy Category C.
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Patients with Reduced CYP2C19 Activity
9 DRUG ABUSE AND DEPENDENCE
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Sedation
17.2 Avoidance of Alcohol and Other CNS Depressants
17.3 Carisoprodol Should Only Be Used for Short-Term Treatment
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Carisoprodol tablets, USP are indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults.
Carisoprodol tablets, USP should only be used for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use has not been established and because acute, painful musculoskeletal conditions are generally of short duration [see Dosage and Administration (2)]. - 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Sedation
Carisoprodol has sedative properties (in the low back pain trials, 13% to 17% of patients who received carisoprodol experienced sedation compared to 6% of patients who received placebo) [see ADVERSE REACTIONS (6.1)] and may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a motor vehicle or operating machinery. There have been post-marketing reports of motor vehicle accidents associated with the use of carisoprodol.
Since the sedative effects of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive, appropriate caution should be exercised with patients who take more than one of these CNS depressants simultaneously.5.2 Drug Dependence, Withdrawal, and Abuse
In the postmarketing experience with carisoprodol, cases of dependence, withdrawal, and abuse have been reported with prolonged use. Most cases of dependence, withdrawal, and abuse occurred in patients who have had a history of addiction or who used carisoprodol in combination with other drugs with abuse potential. However, there have been post-marketing adverse event reports of carisoprodol-associated abuse when used without other drugs with abuse potential. Withdrawal symptoms have been reported following abrupt cessation after prolonged use. To reduce the chance of carisoprodol dependence, withdrawal, or abuse, carisoprodol should be used with caution in addiction-prone patients and in patients taking other CNS depressants including alcohol, and carisoprodol should not be used more than two to three weeks for the relief of acute musculoskeletal discomfort.
Carisoprodol, and one of its metabolites, meprobamate (a controlled substance), may cause dependence [see Clinical Pharmacology (12.3)].5.3 Seizures
There have been postmarketing reports of seizures in patients who received carisoprodol. Most of these cases have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol) [see Overdosage (10)]. -
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect rates observed in practice.
The data described below are based on 910 patients pooled from two double blind, randomized, multicenter, placebo controlled, one-week trials in adult patients with acute, mechanical, lower back pain [see Clinical Studies (14)]. In these studies, patients were treated with 350 mg of carisoprodol, or placebo three times a day and at bedtime for seven days. The mean age was about 41 years old with 54% females and 46% males and 74% Caucasian, 16% Black, 9% Asian, and 2% other.
There were no deaths and there were no serious adverse reactions in these two trials. In these two studies, 2.7% and 5.4%, of patients treated with placebo and 350 mg of carisoprodol, respectively, discontinued due to adverse events; and 0.5% and 1.8% of patients treated with placebo and 350 mg of carisoprodol, respectively, discontinued due to central nervous system adverse reactions.
Table 1 displays adverse reactions reported with frequencies greater than 2% and more frequently than placebo in patients treated with carisoprodol in the two trials described above.Table 1. Patients with Adverse Reactions in Controlled Studies Adverse
ReactionPlacebo (n=560)
n (%)Carisoprodol 350 mg (n=279)
n (%)Drowsiness
31 (6)
47 (17)
Dizziness
11 (2)
19 (7)
Headache
11 (2)
9 (3)
6.2 Postmarketing Experience
The following events have been reported during postapproval use of carisoprodol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: Tachycardia, postural hypotension, and facial flushing [see Overdosage (10)].
Central Nervous System: Drowsiness, dizziness, vertigo, ataxia, tremor, agitation, irritability, headache, depressive reactions, syncope, insomnia, and seizures [see Overdosage (10)].
Gastrointestinal: Nausea, vomiting, and epigastric discomfort.
Hematologic: Leukopenia, pancytopenia. -
7 DRUG INTERACTIONS
7.1 CNS Depressants
The sedative effects of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore, caution should be exercised with patients who take more than one of these CNS depressants simultaneously. Concomitant use of carisoprodol and meprobamate, a metabolite of carisoprodol, is not recommended [see Warnings and Precautions (5.1)].7.2 CYP2C19 Inhibitors and Inducers
Carisoprodol is metabolized in the liver by CYP2C19 to form meprobamate [see Clinical Pharmacology (12.3)]. Co-administration of CYP2C19 inhibitors, such as omeprazole or fluvoxamine, with carisoprodol could result in increased exposure of carisoprodol and decreased exposure of meprobamate. Co-administration of CYP2C19 inducers, such as rifampin or St. John’s Wort, with carisoprodol could result in decreased exposure of carisoprodol and increased exposure of meprobamate. Low dose aspirin also showed an induction effect on CYP2C19. The full pharmacological impact of these potential alterations of exposures in terms of either efficacy or safety of carisoprodol is unknown. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Pregnancy Category C.
There are no data on the use of carisoprodol during human pregnancy. Animal studies indicate that carisoprodol crosses the placenta and results in adverse effects on fetal growth and postnatal survival. The primary metabolite of carisoprodol, meprobamate, is an approved anxiolytic. Retrospective, post-marketing studies do not show a consistent association between maternal use of meprobamate and an increased risk for particular congenital malformations.
Teratogenic effects: Animal studies have not adequately evaluated the teratogenic effects of carisoprodol. There was no increase in the incidence of congenital malformations noted in reproductive studies in rats, rabbits, and mice treated with meprobamate. Retrospective, post-marketing studies of meprobamate during human pregnancy were equivocal for demonstrating an increased risk of congenital malformations following first trimester exposure. Across studies that indicated an increased risk, the types of malformations were inconsistent.
Nonteratogenic effects: In animal studies, carisoprodol reduced fetal weights, postnatal weight gain, and postnatal survival at maternal doses equivalent to 1 to 1.5 times the human dose (based on a body surface area comparison). Rats exposed to meprobamate in-utero showed behavioral alterations that persisted into adulthood. For children exposed to meprobamate in-utero, one study found no adverse effects on mental or motor development or IQ scores. Carisoprodol should be used during pregnancy only if the potential benefit justifies the risk to the fetus.8.2 Labor and Delivery
There is no information about the effects of carisoprodol on the mother and the fetus during labor and delivery.8.3 Nursing Mothers
Very limited data in humans show that carisoprodol is present in breast milk and may reach concentrations two to four times the maternal plasma concentrations. In one case report, a breast-fed infant received about 4 to 6% of the maternal daily dose through breast milk and experienced no adverse effects. However, milk production was inadequate and the baby was supplemented with formula. In lactation studies in mice, female pup survival and pup weight at weaning were decreased. This information suggests that maternal use of carisoprodol may lead to reduced or less effective infant feeding (due to sedation) and/or decreased milk production. Caution should be exercised when carisoprodol is administered to a nursing woman.8.4 Pediatric Use
The efficacy, safety, and pharmacokinetics of carisoprodol in pediatric patients less than 16 years of age have not been established.8.5 Geriatric Use
The efficacy, safety, and pharmacokinetics of carisoprodol in patients over 65 years old have not been established.8.6 Renal Impairment
The safety and pharmacokinetics of carisoprodol in patients with renal impairment have not been evaluated. Since carisoprodol is excreted by the kidney, caution should be exercised if carisoprodol is administered to patients with impaired renal function. Carisoprodol is dialyzable by hemodialysis and peritoneal dialysis.8.7 Hepatic Impairment
The safety and pharmacokinetics of carisoprodol in patients with hepatic impairment have not been evaluated. Since carisoprodol is metabolized in the liver, caution should be exercised if carisoprodol is administered to patients with impaired hepatic function.8.8 Patients with Reduced CYP2C19 Activity
Patients with reduced CYP2C19 activity have higher exposure to carisoprodol. Therefore, caution should be exercised in administration of carisoprodol to these patients [see Clinical Pharmacology (12.3)]. -
9 DRUG ABUSE AND DEPENDENCE
Carisoprodol is a controlled substance [see Warnings and Precautions (5.2)]. Discontinuation of carisoprodol in animals or in humans after chronic administration can produce withdrawal signs, and there are published case reports of human carisoprodol dependence.
In vitro studies demonstrate that carisoprodol elicits barbiturate-like effects. Animal behavioral studies indicate that carisoprodol produces rewarding effects. Monkeys self administer carisoprodol. Drug discrimination studies using rats indicate that carisoprodol has positive reinforcing and discriminative effects similar to barbital, meprobamate, and chlordiazepoxide. -
10 OVERDOSAGE
Overdosage of carisoprodol commonly produces CNS depression. Death, coma, respiratory depression, hypotension, seizures, delirium, hallucinations, dystonic reactions, nystagmus, blurred vision, mydriasis, euphoria, muscular incoordination, rigidity, and/or headache have been reported with carisoprodol overdosage. Many of the carisoprodol overdoses have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol). The effects of an overdose of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) can be additive even when one of the drugs has been taken in the recommended dosage. Fatal accidental and non-accidental overdoses of carisoprodol have been reported alone or in combination with CNS depressants.
Treatment of Overdosage: Basic life support measures should be instituted as dictated by the clinical presentation of the carisoprodol overdose. Induced emesis is not recommended due to the risk of CNS and respiratory depression, which may increase the risk of aspiration pneumonia. Gastric lavage should be considered soon after ingestion (within one hour). Circulatory support should be administered with volume infusion and vasopressor agents if needed. Seizures should be treated with intravenous benzodiazepines and the reoccurrence of seizures may be treated with phenobarbital. In cases of severe CNS depression, airway protective reflexes may be compromised and tracheal intubation should be considered for airway protection and respiratory support.
The following types of treatment have been used successfully with an overdose of meprobamate, a metabolite of carisoprodol: activated charcoal (oral or via nasogastric tube), forced diuresis, peritoneal dialysis, and hemodialysis (carisoprodol is also dialyzable). Careful monitoring of urinary output is necessary and overhydration should be avoided. Observe for possible relapse due to incomplete gastric emptying and delayed absorption. For more information on the management of an overdose of carisoprodol, contact a Poison Control Center. -
11 DESCRIPTION
Carisoprodol tablets are available as 350 mg round, white tablets. Carisoprodol USP is a white, crystalline powder, having a mild, characteristic odor and a bitter taste. It is slightly soluble in water; freely soluble in alcohol, in chloroform, and in acetone; and its solubility is practically independent of pH. Carisoprodol is present as a racemic mixture. Chemically, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate and the molecular formula is C12H24N2O4, with a molecular weight of 260.33. The structural formula is:
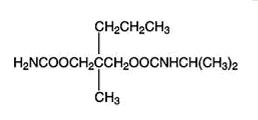
Other ingredients in the carisoprodol tablets include pregelatinized starch, crospovidone, povidone, microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of carisoprodol in relieving discomfort associated with acute painful musculoskeletal conditions has not been clearly identified.
In animal studies, muscle relaxation induced by carisoprodol is associated with altered interneuronal activity in the spinal cord and in the descending reticular formation of the brain.12.2 Pharmacodynamics
Carisoprodol is a centrally acting skeletal muscle relaxant that does not directly relax skeletal muscles.
A metabolite of carisoprodol, meprobamate, has anxiolytic and sedative properties. The degree to which these properties of meprobamate contribute to the safety and efficacy of carisoprodol is unknown.12.3 Pharmacokinetics
The pharmacokinetics of carisoprodol and its metabolite meprobamate were studied in a crossover study of 24 healthy subjects (12 male and 12 female) who received single doses of 350 mg carisoprodol (see Table 2). The Cmax of meprobamate was 2.5 ± 0.5 mcg/mL (mean ± SD) after administration of a single 350 mg dose of carisoprodol, which is approximately 30% of the Cmax of meprobamate (approximately 8 mcg/mL) after administration of a single 400 mg dose of meprobamate.Table 2. Pharmacokinetic Parameters of Carisoprodol and Meprobamate (Mean ± SD, n=24) 350 mg Carisoprodol Carisoprodol
Cmax (mcg/mL)
1.8 ± 1
AUCinf (mcg*hr/mL)
7 ± 5
Tmax (hr)
1.7 ± 0.8
T1/2 (hr)
2 ± 0.5
Meprobamate
Cmax (mcg/mL)
2.5 ± 0.5
AUCinf (mcg*hr/mL)
46 ± 9
Tmax (hr)
4.5 ± 1.9
T1/2 (hr)
9.6 ± 1.5
Absorption: Absolute bioavailability of carisoprodol has not been determined. The mean time to peak plasma concentrations (Tmax) of carisoprodol was approximately 1.5 to 2 hours. Co-administration of a high-fat meal with carisoprodol (350 mg tablet) had no effect on the pharmacokinetics of carisoprodol. Therefore, carisoprodol may be administered with or without food.
Metabolism: The major pathway of carisoprodol metabolism is via the liver by cytochrome enzyme CYP2C19 to form meprobamate. This enzyme exhibits genetic polymorphism (see Patients with Reduced CYP2C19 Activity below).
Elimination: Carisoprodol is eliminated by both renal and non-renal routes with a terminal elimination half-life of approximately 2 hours. The half-life of meprobamate is approximately 10 hours.
Gender: Exposure of carisoprodol is higher in female than in male subjects (approximately 30 to 50% on a weight adjusted basis). Overall exposure of meprobamate is comparable between female and male subjects.
Patients with Reduced CYP2C19 Activity: Carisoprodol should be used with caution in patients with reduced CYP2C19 activity. Published studies indicate that patients who are poor CYP2C19 metabolizers have a 4-fold increase in exposure to carisoprodol, and concomitant 50% reduced exposure to meprobamate compared to normal CYP2C19 metabolizers. The prevalence of poor metabolizers in Caucasians and African Americans is approximately 3 to 5% and in Asians is approximately 15 to 20%. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed to evaluate the carcinogenic potential of carisoprodol.
Carisoprodol was not formally evaluated for genotoxicity. In published studies, carisoprodol was mutagenic in the in vitro mouse lymphoma cell assay in the absence of metabolizing enzymes, but was not mutagenic in the presence of metabolizing enzymes. Carisoprodol was clastogenic in the in vitro chromosomal aberration assay using Chinese hamster ovary cells with or without the presence of metabolizing enzymes. Other types of genotoxic tests resulted in negative findings. Carisoprodol was not mutagenic in the Ames reverse mutation assay using S. typhimurium strains with or without metabolizing enzymes, and was not clastogenic in an in vivo mouse micronucleus assay of circulating blood cells.
Carisoprodol was not formally evaluated for effects on fertility. Published reproductive studies of carisoprodol in mice found no alteration in fertility although an alteration in reproductive cycles characterized by a greater time spent in estrus was observed at a carisoprodol dose of 1200 mg/kg/day. In a 13-week toxicology study that did not determine fertility, mouse testes weight and sperm motility were reduced at a dose of 1200 mg/kg/day. In both studies, the no effect level was 750 mg/kg/day, corresponding to approximately 2.6 times the human equivalent dosage of 350 mg four times a day, based on a body surface area comparison.
The significance of these findings for human fertility is not known. -
14 CLINICAL STUDIES
The safety and efficacy of carisoprodol for the relief of acute, idiopathic mechanical low back pain was evaluated in two, 7-day, double blind, randomized, multicenter, placebo controlled, U.S. trials (Studies 1 and 2). Patients had to be 18 to 65 years old and had to have acute back pain (≤ 3 days of duration) to be included in the trials. Patients with chronic back pain; at increased risk for vertebral fracture (e.g., history of osteoporosis); with a history of spinal pathology (e.g., herniated nucleus pulposis, spondylolisthesis or spinal stenosis); with inflammatory back pain, or with evidence of a neurologic deficit were excluded from participation. Concomitant use of analgesics (e.g., acetaminophen, NSAIDs, tramadol, opioid agonists), other muscle relaxants, botulinum toxin, sedatives (e.g., barbiturates, benzodiazepines, promethazine hydrochloride), and anti-epileptic drugs was prohibited.
In Study 1, patients were randomized to one of two treatment groups (i.e., carisoprodol 350 mg or placebo). In the study, patients received study medication three times a day and at bedtime for seven days.
The primary endpoints were the relief from starting backache and the global impression of change, as reported by patients, on Study Day 3. Both endpoints were scored on a 5-point rating scale from 0 (worst outcome) to 4 (best outcome) in both studies. The primary statistical comparison was between the carisoprodol 250 mg and placebo groups in both studies.
The proportion of patients who used concomitant acetaminophen, NSAIDs, tramadol, opioid agonists, other muscle relaxants, and benzodiazepines was similar in the treatment groups.
The results for the primary efficacy evaluations in the acute, low back pain studies are presented in Table 3.Table 3. Results of the Primary Efficacy Endpointsa in Study 1 Study Parameter Placebo Carisoprodol 350 mg a The primary efficacy endpoints (Relief from Starting Backache and Global Impression of Change) were assessed by the patients on Study Day 3. These endpoints were scored on a 5-point rating scale from 0 (worst outcome) to 4 (best outcome).
b Mean is the least squared mean and SE is the standard error of the mean.
1Number of Patients
n=269
n=273
Relief from Starting Backache, Mean (SE)b
1.4 (0.1)
1.8 (0.1)
Difference between Carisoprodol and Placebo, Mean (SE)b (95% CI)
0.4
(0.2, 0.6)
Global Impression of Change, Mean (SE)b
1.9 (0.1)
2.2 (0.1)
Difference between Carisoprodol and Placebo, Mean (SE)b (95% CI)
0.3
(0.1, 0.4)
Patients treated with carisoprodol experienced improvement in function as measured by the Roland-Morris Disability Questionnaire (RMDQ) score on Days 3 and 7. - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Patients should be advised to contact their physician if they experience any adverse reactions to carisoprodol.17.1 Sedation
Patients should be advised that carisoprodol may cause drowsiness and/or dizziness, and has been associated with motor vehicle accidents. Patients should be advised to avoid taking carisoprodol before engaging in potentially hazardous activities such as driving a motor vehicle or operating machinery [see Warnings and Precautions (5.1)].17.2 Avoidance of Alcohol and Other CNS Depressants
Patients should be advised to avoid alcoholic beverages while taking carisoprodol and to check with their doctor before taking other CNS depressants such as benzodiazepines, opioids, tricyclic antidepressants, sedating antihistamines, or other sedatives [see Warnings and Precautions (5.1)].17.3 Carisoprodol Should Only Be Used for Short-Term Treatment
Patients should be advised that treatment with carisoprodol should be limited to acute use (up to two or three weeks) for the relief of acute, musculoskeletal discomfort. In the post-marketing experience with carisoprodol, cases of dependence, withdrawal, and abuse have been reported with prolonged use. If the musculoskeletal symptoms still persist, patients should contact their healthcare provider for further evaluation.
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured for:
Aurobindo Pharma USA, Inc.
2400 Route 130 North
Dayton, NJ 08810
Manufactured by:
Aurobindo Pharma Limited
Hyderabad–500 072, India
Revised: 02/2012 - PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 350 mg (100 Tablet Bottle)
-
INGREDIENTS AND APPEARANCE
CARBAMAZEPINE
carbamazepine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 33261-676(NDC:51672-4005) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMAZEPINE (UNII: 33CM23913M) (CARBAMAZEPINE - UNII:33CM23913M) CARBAMAZEPINE 200 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIETHYL PHTHALATE (UNII: UF064M00AF) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code TARO;11 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 33261-676-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074649 10/03/1996 Labeler - Aidarex Pharmaceuticals LLC (801503249)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
