METRONIDAZOLE tablet, film coated, extended release
Metronidazole by
Drug Labeling and Warnings
Metronidazole by is a Prescription medication manufactured, distributed, or labeled by Alembic Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING
Metronidazole has been shown to be carcinogenic in mice and rats. (See PRECAUTIONS.)
Unnecessary use of the drug should be avoided. Its use should be reserved for conditions described in the INDICATIONS AND USAGE section below. -
DESCRIPTION
Metronidazole is an oral synthetic antiprotozoal and antibacterial agent, 2-methyl-5-nitroimidazole-1-ethanol, which has the following structural formula:
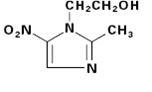
Metronidazole extended-release tablets, 750 mg contain 750 mg of metronidazole USP. Inactive ingredients include Lactose monohydrate, Polyacrylate Dispersion 30 Percent, Povidone, Magnesium Stearate, Polysorbate 80, Hypromellose, Titanium dioxide, Polydextrose, Triacetin, Polyethylene glycol and yellow iron oxide.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
Disposition of metronidazole in the body is similar for both oral and intravenous dosage forms, with an average elimination half-life in healthy humans of 8 hours.
The major route of elimination of metronidazole and its metabolites is via the urine (60%to 80%of the dose), with fecal excretion accounting for 6%to 15%of the dose. The metabolites that appear in the urine result primarily from side-chain oxidation [1-(β-hydroxyethyl)-2-hydroxymethyl-5-nitroimidazole and 2-methyl-5-nitroimidazole-1-yl-acetic acid] and glucuronide conjugation, with unchanged metronidazole accounting for approximately 20%of the total. Renal clearance of metronidazole is approximately 10 mL/min/1.73m2. 1
Metronidazole extended-release tablets, 750 mgcontain 750 mg of metronidazole in an extended release formulation which allows for once-daily dosing. The steady state pharmacokinetics were determined in 24 healthy adult female subjects with a mean ± SD age of 28.8 ± 8.8 years (range: 19 - 46).2 The pharmacokinetic parameters of metronidazole after administration of Metronidazole extended-release tablets, 750 mg under fed and fasting conditions are summarized in the following table.
Steady State Pharmacokinetic Parameters of Metronidazole after 750 mg of Metronidazole extended-release tablets Given Once a Day for 7 Days
______________________________________________________________________________________________
Metronidazole extended-release tablets, 750 mg daily
Mean ± SD (N = 24)
Parameter fed fasted
AUC(0-24) (⛤g·hr / mL) 211 ± 60.0 198 ± 75.3
Cmax (⛤g/mL) 19.4 ± 4.7 12.5 ± 4.8
Cmin (⛤g/mL) 3.4 ± 2.0 4.2 ± 2.2
Tmax (hrs) 4.6 ± 2.4 6.8 ± 2.8
T1/2 (hrs) 7.4 ± 1.6 8.7 ± 2.2
Relative to the fasting state, the rate of metronidazole absorption from the extended-release tablet is increased in the fed state resulting in alteration of the extended-release characteristics.
Decreased renal function does not alter the single-dose pharmacokinetics of metronidazole. However, plasma clearance of metronidazole is decreased in patients with decreased liver function.Microbiology
Metronidazole exerts an antimicrobial effect in an anaerobic environment by the following possible mechanism: Once metronidazole enters the organism, the drug is reduced by intracellular electron transport proteins. Because of this alteration to the metronidazole molecule, a concentration gradient is maintained which promotes the drug’s intracellular transport. Presumably, free radicals are formed which, in turn, react with cellular components resulting in death of the microorganism.
The following in vitro data are available, but their clinical significance is unknown:
Metronidazole exhibits in vitro minimal inhibitory concentrations (MIC’s) of 8 ⛤g/mL or less against most (≥90%) strains of the following microorganisms; however, the safety and effectiveness of metronidazole in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Gram-positive anaerobes:
Clostridium species
Eubacterium species
Peptococcus niger
Peptostreptococcus species
Gram-negative anaerobes:
Bacteroides fragilis group (B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B. vulgatus)
Fusobacterium species
Prevotella species (P. bivia, P. buccae, P. disiens)
Porphyromonas species
Protozoal parasites:
Entamoeba histolytica
Trichomonas vaginalis
Metronidazole has shown minimal to no activity against clinically relevant facultative anaerobes or obligate aerobes. Metronidazole has minimal activity against Lactobacillus spp and other aerobic microorganisms commonly isolated from the vaginal tract.
Susceptibility Tests:
Dilution techniques:
Quantitative methods that are used to determine minimum inhibitory concentrations provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. For anaerobic bacteria, the susceptibility to metronidazole can be determined by the reference agar dilution method or by alternate standardized test methods.3 The MIC values obtained should be interpreted according to the following criteria:
MIC (⛤g/mL) Interpretation
≤ 8 Susceptible (S)
16 Intermediate (I)
≥32 Resistant (R)
For protozoal parasites: Standardized tests do not exist for use in clinical microbiology laboratories.
A report of “Susceptible” indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in the blood. A report of “Intermediate” indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. Standard metronidazole powder should provide the following MIC values:
Microorganism MIC (⛤g/mL)
Bacteroides fragilis ATCC 25285 0.25 – 1.0
Bacteroides thetaiotaomicron ATCC 29741 0.5 – 2.0 -
INDICATIONS AND USAGE
Bacterial Vaginosis (BV). Metronidazole extended-release tablets, 750 mg are indicated in the treatment of women with BV.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Metronidazole extended-release tablet and other antibacterial drugs, Metronidazole extended-release tablet should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. -
CONTRAINDICATIONS
Metronidazole extended-release tablets, 750 mg are contraindicated in patients with a prior history of hypersensitivity to metronidazole or other nitroimidazole derivatives.
Metronidazole extended-release tablet, like other formulations of metronidazole-containing products, is contraindicated during the first trimester of pregnancy. (See PRECAUTIONS.) -
WARNINGS
Central and Peripheral Nervous System Effects:Convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity, have been reported in patients treated with metronidazole. The appearance of abnormal neurologic signs demands the prompt discontinuation of metronidazole therapy. Metronidazole should be administered with caution to patients with central nervous system diseases.
-
PRECAUTIONS
General
Patients with severe hepatic disease metabolize metronidazole slowly, with resultant accumulation of metronidazole and its metabolites in the plasma. Accordingly, for such patients, doses below those usually recommended should be administered cautiously. Known or previously unrecognized candidiasis may present more prominent symptoms during therapy with metronidazole and requires treatment with a candidacidal agent.
Prescribing Metronidazole extended-release tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.Information for patients
Alcoholic beverages should be avoided while taking metronidazole and for at least three days afterward. (See Drug interactions.)
Patients should be counseled that antibacterial drugs including Metronidazole extended-release tablet should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Metronidazole extended-release tablet is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Metronidazole extended-release tablet or other antibacterial drugs in the future.Laboratory tests
Metronidazole is a nitroimidazole and should be used with caution in patients with evidence of or history of blood dyscrasia. A mild leukopenia has been observed during its administration; however, no persistent hematologic abnormalities attributable to metronidazole have been observed in clinical studies. Total and differential leukocyte counts should be made before and after re-treatments.
Drug interactions
Metronidazole has been reported to potentiate the anticoagulant effect of warfarin and other oral coumarin anticoagulants, resulting in a prolongation of prothrombin time. This possible drug interaction should be considered when metronidazole is prescribed for patients on this type of anticoagulant therapy.
The simultaneous administration of drugs that induce microsomal liver enzymes, such as phenytoin or phenobarbital, may accelerate the elimination of metronidazole, resulting in reduced plasma levels; impaired clearance of phenytoin has been reported.
The simultaneous administration of drugs that decrease microsomal liver enzyme activity, such as cimetidine, may prolong the half-life and decrease plasma clearance of metronidazole. In patients stabilized on relatively high doses of lithium, short-term metronidazole therapy has been associated with elevation of serum lithium and, in a few cases, signs of lithium toxicity. Serum lithium and serum creatinine levels should be obtained several days after beginning metronidazole to detect any increase that may precede clinical symptoms of lithium intoxication.
Alcoholic beverages should not be consumed during metronidazole therapy and for at least three days afterward because abdominal cramps, nausea, vomiting, headaches, and flushing may occur.
Psychotic reactions have been reported in alcoholic patients who are using metronidazole and disulfiram concurrently. Metronidazole should not be given to patients who have taken disulfiram within the last 2 weeks.Drug/Laboratory test interactions
Metronidazole may interfere with certain types of determinations of serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides, and hexokinase glucose. Values of zero may be observed. All of the assays in which interference has been reported involve enzymatic coupling of the assay to oxidation-reduction of nicotinamide adenine dinucleotide (NAD+ ↔ NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7.
Carcinogenesis, mutagenesis, impairment of fertility
Pulmonary tumors have been observed in all six reported studies in the mouse, including one study in which the animals were dosed on an intermittent schedule (administration during every fourth week only).
Malignant liver tumors were increased in male mice treated at approximately 1500 mg/m2. This dose is approximately 3 times the recommended dose.
Malignant lymphomas and pulmonary neoplasms are also increased with lifetime feeding of the drug to mice (published data).
Mammary and hepatic tumors were increased among female rats administered oral metronidazole compared to concurrent controls.
Two lifetime tumorigenicity studies in hamsters have been performed and reported to be negative.
Metronidazole has shown mutagenic activity in in vitro assay systems including the Ames test. Studies in mammals in vivo have failed to demonstrate a potential for genetic damage. Fertility studies have been performed in mice at doses up to six times the maximum recommended human dose based on mg/m2 and have revealed no evidence of impaired fertility.Pregnancy
Teratogenic effects: Pregnancy Category B.
Metronidazole extended-release tablet has not been studied in pregnant women. Since metronidazole crosses the placental barrier and enters the fetal circulation rapidly, it should not be administered to pregnant patients during the first trimester. No fetotoxicity was observed when metronidazole was administered orally to pregnant mice at 60 mg/ m2/day, which is approximately 10%of the human dose when expressed as mg/m2. However, in a single small study where the drug was administered intraperitoneally, some intrauterine deaths were observed. The relationship of these findings to the drug is unknown. There are, however, no adequate and well-controlled studies in pregnant women. (See CONTRAINDICATIONS.)
Because animal reproduction studies are not always predictive of human response, and because metronidazole is a carcinogen in rodents, this drug should be used during pregnancy only if clearly needed.Nursing mothers
Since metronidazole is secreted in human milk in concentrations similar to those found in plasma, and since tumors were increased in rats and mice treated with metronidazole, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric use
Safety and effectiveness of this dosage form of metronidazole in pediatric patients have not been established.
Geriatric use
Decreased renal function does not alter the single dose pharmacokinetics of metronidazole. However, plasma clearance of metronidazole is decreased in patients with decreased liver function. Therefore, in elderly patients, monitoring of serum levels may be necessary to adjust the metronidazole dosage accordingly.
-
ADVERSE REACTIONS
In two multicenter clinical trials, a total of 270 patients received 750 mg Metronidazole extended-release tablets orally once daily for 7 days, and 287 were treated with a comparator agent administered intravaginally once daily for 7 days. (See CLINICAL STUDIES.) 4, 5
Most adverse events were described as being of mild or moderate severity. Among patients taking Metronidazole extended-release tablet who reported headaches, 10%considered them severe, and less than 2%of reported episodes of nausea were considered severe. Metallic taste was reported by 9%of patients taking Metronidazole extended-release tablet.
Adverse events reported at ≥2%incidence for either treatment group, irrespective of treatment causality, are summarized in the table below.
Adverse Events
(≥2%Incidence Rate) – Irrespective of Treatment Causality
Metronidazole Extended- Vaginal Preparation
Release Tablets 7 days
(N=267) (N=285)
Headache 48 (18%) 44 (15%)
Vaginitis 39 (15%) 32 (12%)
Nausea 28 (10%) 8 (3%)
Taste Perversion (metallic taste) 23 (9%) 1 (0%)
Infection Bacterial 19 (7%) 17 (6%)
Influenza-like Symptoms 17 (6%) 20 (7%)
Pruritus Genital 14 (5%) 25 (9%)
Abdominal Pain 10 (4%) 13 (5%)
Dizziness 11 (4%) 3 (1%)
Diarrhea 11 (4%) 3 (1%)
Upper Respiratory Tract Infection 11 (4%) 10 (4%)
Rhinitis 12 (4%) 10 (4%)
Sinusitis 7 (3%) 6 (2%)
Urine Abnormal 7 (3%) 4 (1%)
Pharyngitis 8 (3%) 4 (1%)
Dysmenorrhea 9 (3%) 7 (2%)
Moniliasis 9 (3%) 8 (3%)
Mouth Dry 5 (2%) 2 (1%)
Urinary Tract Infection 6 (2%) 16 (6%)
Vulvovaginal candidiasis is a recognized consequence of treatment with many anti-infective agents. In these multicenter clinical trials, there were no statistically significant differences in the incidence rates of yeast vaginitis for groups of patients treated with Metronidazole extended-release tablet or the vaginal comparator.
The following reactions have also been reported during treatment with metronidazole:
Central Nervous System: The most serious adverse reactions reported in patients treated with metronidazole have been convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity. Since persistent peripheral neuropathy has been reported in some patients receiving prolonged administration of metronidazole, patients should be specifically warned about these reactions and should be told to stop the drug and report immediately to their physicians if any neurologic symptoms occur. In addition, patients have reported dizziness, vertigo, incoordination, ataxia, confusion, dysarthria, irritability, depression, weakness, and insomnia. (See WARNINGS.)
Gastrointestinal: The most common adverse reactions reported have been referable to the gastrointestinal tract, particularly nausea reported by about 12%of patients, sometimes accompanied by headache, anorexia, and occasionally vomiting, diarrhea, epigastric distress, and abdominal cramping. Constipation has also been reported.
Furry tongue, glossitis, and stomatitis have occurred; these may be associated with a sudden overgrowth of Candida which may occur during therapy. Rare cases of pancreatitis, which generally abated on withdrawal of the drug, have been reported.
Hematopoietic: Reversible neutropenia (leukopenia); rarely, reversible thrombocytopenia.
Cardiovascular: Flattening of the T-wave may be seen in electrocardiographic tracings.
Hypersensitivity: Urticaria, erythematous rash, Stevens-Johnson Syndrome, toxic epidermal necrolysis, flushing, nasal congestion, dryness of the mouth (or vagina or vulva), and fever.
Renal: Dysuria, cystitis, polyuria, incontinence, and a sense of pelvic pressure. Instances of darkened urine have been reported by approximately one patient in 100,000. Although the pigment which is probably responsible for this phenomenon has not been positively identified, it is almost certainly a metabolite of metronidazole and seems to have no clinical significance.
Other: Proliferation of Candida in the vagina, dyspareunia, decrease of libido, proctitis, and fleeting joint pains sometimes resembling “serum sickness”. If patients receiving metronidazole drink alcoholic beverages, they may experience abdominal distress, nausea, vomiting, flushing, or headache. A modification of the taste of alcoholic beverages has also been reported.
Patients with Crohn’s disease are known to have an increased incidence of gastrointestinal and certain extraintestinal cancers. There have been some reports in the medical literature of breast and colon cancer in Crohn’s disease patients who have been treated with metronidazole at high doses for extended periods of time. A cause and effect relationship has not been established. Crohn’s disease is not an approved indication for metronidazole extended-release tablets, 750 mg. -
OVERDOSAGE
Single oral doses of metronidazole, up to 15 g, have been reported in suicide attempts and accidental overdoses. Symptoms reported include nausea, vomiting, and ataxia.
Oral metronidazole has been studied as a radiation sensitizer in the treatment of malignant tumors. Neurotoxic effects, including seizures and peripheral neuropathy, have been reported after 5 to 7 days of doses of 6 g to 10.4 g every other day.
Treatment: There is no specific antidote for metronidazole overdose; therefore, management of the patient should consist of symptomatic and supportive therapy. -
DOSAGE AND ADMINISTRATION
Bacterial Vaginosis:
Seven-day course of treatment – 750 mg once daily by mouth for seven consecutive days.
Metronidazole extended-release tablets, 750 mg should be taken under fasting conditions, at least one hour before or two hours after meals. The optimum extended-release characteristics of Metronidazole extended-release tablets, 750 mg are obtained when the drug is taken under fasting conditions. (See CLINICAL PHARMACOLOGY- Pharmacokinetics.)
Pregnant patients should not be treated during the first trimester. (See CONTRAINDICATIONSand PRECAUTIONS.)
Patients with severe hepatic disease metabolize metronidazole slowly, with resultant accumulation of metronidazole and its metabolites in the plasma. Accordingly, for such patients, doses below those usually recommended should be administered cautiously. Close monitoring of plasma metronidazole levels6 and toxicity is recommended.
The dose of metronidazole should not be specifically reduced in anuric patients because accumulated metabolites may be rapidly removed by dialysis.
In elderly patients, the pharmacokinetics of metronidazole may be altered and therefore, monitoring of serum levels may be necessary to adjust the metronidazole dosage accordingly. -
HOW SUPPLIED
Metronidazole extended-release tablets, 750 mg are Yellow, oblong, biconvex film coated tablets debossed with L106 on one side and plain on other side, and supplied as:
NDC Number Size
46708-021-30 Bottle of 30
46708-021-50 Bottle of 50
46708-021-31 Bottle of 100
46708-021-71 Bottle of 500
Storage and Stability:Store in a dry place at 25°C (77°F); excursions permitted to 15°–30°C (59°–86°F). [See USP Controlled Room Temperature.] Dispense in a well-closed container with a child-resistant closure.
-
CLINICAL STUDIES
BV is a clinical syndrome that results from a replacement of the normal, Lactobacillus-dominant flora with several other organisms including Gardnerella vaginalis, Mobiluncus spp, Mycoplasma hominis and anaerobes (Peptostreptococcus spp and Bacteroides spp).
Metronidazole extended-release tablet was studied in patients with BV in two randomized, multicenter, well-controlled, investigator blind clinical trials.4, 5 A total of 557 otherwise healthy non pregnant patients with BV were randomized to treatment with Metronidazole extended-release tablet once a day for 7 days (n = 270) or 2%clindamycin vaginal cream one applicator full (5 grams) once a day for 7 days (n = 287).
The primary efficacy endpoint for each treatment regimen was defined as clinical cure assessed at 28 – 32 days post-therapy. Clinical cure was defined as a return to normal of the vaginal pH (≤4.5), absence of a “fishy” amine odor, and absence of clue cells. The study results are presented in the table below:
Clinical Cure Rates at One Month
Metronidazole Extended- 2%clindamycin cream
Release Tablets
%(n/N) %(n/N)
Study 1 61%(77/126) 59%(80/135)
Study 2 62%(74/119)* 43%(50/117)
*p<0.05 versus clindamycin cream
At one month post-therapy the pH of the vagina returned to normal earlier and in a greater percentage of patients in the Metronidazole extended-release tablet treatment group when compared to the 2%clindamycin vaginal cream group; 72%vs 65%, respectively. Likewise, Metronidazole extended-release tablet restored the normal Lactobacillus-predominant vaginal flora in a larger percentage of patients at one month post-therapy when compared to the 2%clindamycin treated group; 74%vs 63%, respectively. -
REFERENCES
1. Salas-Herrera IG, Pearson RM, Johnston A, and Turner P. Concentration of metronidazole in cervical mucus and serum after single and repeated oral doses. J Antimicrobial Chemotherapy 1991; 28:283-289. 2. Metronidazole modified-release tablet multiple-dose bioequivalency study (fed/fasting). G.D. Searle & Co., Protocol No. S13-94-02-014; Report No. S13-95-06-014, 11 July 1995. 3. National Committee for Clinical Laboratory Standards, Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria—Third Edition. Approved Standard NCCLS Document M11-A3, Vol. 13, No. 26, NCCLS, Villanova, PA, December, 1993. 4. Integrated clinical and statistical report for the treatment of bacterial vaginosis with metronidazole modified release tablet—a dose duration study. G.D. Searle & Co., Protocol No. N13-95-02-015; Report No. N13-96-06-015, 19 Nov 1996. 5. Integrated clinical and statistical report for the treatment of bacterial vaginosis with metronidazole modified release tablet. G.D. Searle & Co., Protocol No. N13-95-02-017; Report No. N13-96-06-017, 11 Nov 1996. 6. Ralph ED, Kirby WMM. Bioassay of metronidazole with either anaerobic or aerobic incubation. J Infect Dis 1975; 132:587-591 or Gulaid et al. Determination of metronidazole and its major metabolites in biological fluids by high pressure liquid chromatography. Br J Clin Pharmacol 1978; 6:430-432.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Rx only

Manufactured by:
Alembic Pharmaceuticals Limited (Formulation Division),
Village Panelav, P. O. Tajpura, Near Baska,
Taluka-Halol, Panchmahal, Gujarat, India.
Revised: 06/2011 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Each extended-release film coated tablet contains Metronidazole USP 750 mg
NDC: 46708-021-30
30 Tablets HDPE Bottle Pack

-
INGREDIENTS AND APPEARANCE
METRONIDAZOLE
metronidazole tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-021 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METRONIDAZOLE (UNII: 140QMO216E) (METRONIDAZOLE - UNII:140QMO216E) METRONIDAZOLE 750 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYDEXTROSE (UNII: VH2XOU12IE) TRIACETIN (UNII: XHX3C3X673) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score no score Shape OVAL (Oblong, biconvex) Size 19mm Flavor Imprint Code L106 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-021-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2010 2 NDC: 46708-021-50 50 in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2010 3 NDC: 46708-021-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2010 4 NDC: 46708-021-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090222 05/07/2010 Labeler - Alembic Pharmaceuticals Limited (650574663) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 650574671 MANUFACTURE(46708-021)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.