Omeprazole Delayed Release Tablets, 20 mg
Omeprazole by
Drug Labeling and Warnings
Omeprazole by is a Otc medication manufactured, distributed, or labeled by Dexcel Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
OMEPRAZOLE DELAYED RELEASE- omeprazole tablet, delayed release
Dexcel Ltd.
----------
Omeprazole Delayed Release Tablets, 20 mg
Omeprazole Delayed Release Tablets, 20 mg Drug Facts
Active ingredient (in each tablet)
Omeprazole delayed-release tablet, 20 mg
Use
- treats frequent heartburn (occurs 2 or more days a week)
- not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
Warning
Allergy alert: Do not use if you are allergic to omeprazole
Do not use if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness or breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are taking
- clopidogrel bisulfate (anti-blood clotting medicine)
- warfarin (blood-thinning medicine)
- prescription antifungal or anti-yeast medicines
- diazepam (anxiety medicine)
- digoxin (heart medicine)
- tacrolimus (immune system medicine)
- atazanavir (medicine for HIV infection)
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
- swallow 1 tablet with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 tablet a day
- do not chew or crush the tablets
- do not crush tablets in food
- do not use for more than 14 days unless directed by your doctor
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor
Other information
- read the directions, warnings, and package insert before use
- keep the carton and package insert. They contain important information.
- store at 20-25°C (68-77°F)
- keep product out of high heat and humidity
- protect product from moisture
Inactive ingredients carnauba wax, ferric oxide red, ferric oxide yellow, hypromellose, hypromellose acetate succinate, lactose monohydrate, monoethanolamine, propylene glycol, sodium lauryl sulfate, sodium starch glycolate, sodium stearate, sodium stearyl fumarate, talc, titanium dioxide, triethyl citrate
Inner Carton Label
Treats Frequent Heartburn!
Occurring 2 or More Days a Week
Omeprazole
delayed release tablets 20 mg
acid reducer
14 TABLETS
One 14-day course of treatment

Blister Label
Omeprazole DR Tablets
20 mg
Acid reducer
Push tablet through foil.
Dexcel Ltd.
1 Dexcel St. Or Akiva
30600, Israel
Do not chew or crush tablets
Do not crush tablets in food

Bottle Label
NOT FOR RESALE
Treats Frequent Heartburn!
Occurring 2 or More Days a Week
Omeprazole Delayed
Release Tablets 20 mg
Acid Reducer
14 Tablets
One 14-day course of treatment
DO NOT USE IF PRINTED SEAL UNDER CAP IS BROKEN OR MISSING
Manufactured by:
Dexcel® Ltd.
1 Dexcel St., Or Akiva
30600, Israel
Made in Israel



Patient Package Insert
Omeprazole Delayed Release Tablets 20 mg
Acid Reducer
Please read all of this package insert before taking Omeprazole Delayed Release Tablets 20 mg. Save this to read, as you need.
How Omeperazole Delayed Release Tablets 20 mg Work For Your Frequent Heartburn
Omeprazole Delayed Release Tablets 20 mg work differently from other heartburn products, such as antacids and other acid reducers. Omeprazole Delayed Release Tablets 20 mg stop acid production at the source - the acid pump that produces stomach acid. Omeprazole Delayed Release Tablets 20 mg are to be used once a day (every 24 hours), every day for 14 days.
What to Expect When Using Omeprazole Delayed Release Tablets 20 mg
Omeprazole Delayed Release Tablets 20 mg are a different type of medicine from antacids and other acid reducers. Omeprazole Delayed Release Tablets 20 mg may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours. Make sure you take the entire 14 days of dosing to treat your frequent heartburn.
Safety Record
For years, doctors have prescribed omeprazole to treat acid-related conditions in millions of people safely.
Who Should Take Omeprazole Delayed Release Tablets 20 mg
This product is for adults (18 years and older) with frequent heartburn - when you have heartburn 2 or more days a week.
- Omeprazole Delayed Release Tablets 20 mg are not intended for those who have heartburn infrequently, one episode of heartburn a week or less, or for those who want immediate relief or heartburn.
14-Day Course of Treatment
- Swallow 1 tablet with a glass of water before eating in the morning.
- Take every day for 14 days.
- Do not take more than 1 tablet a day
- Do not chew or crush the tablets.
- Do not crush tablets in food.
- Do not use for more than 14 days unless directed by your doctor.
When to Take Omeprazole Delayed Release Tablets 20 mg Again
You may repeat a 14-day course of therapy every 4 months.
When to Talk to Your Doctor
Do not take for more than 14 days or more often than every 4 months unless directed by a doctor.
Warnings and When to Ask Your Doctor
Allergy alert: Do not use if you are allergic to omeprazole
Do not use if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- clopidogrel bisulfate (anti-blood clotting medicine)
- warfarin (blood-thinning medicine)
- prescription antifungal or anti-yeast medicines
- diazepam (anxiety medicine)
- digoxin (heart medicine)
- tacrolimus (immune system medicine)
- atazanavir (medicine for HIV infection)
- your heartburn continues or worsens
- you need to take this product for more than 14 days
- you need to take more than 1 course of treatment every 4 months
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Tips for Managing Heartburn
- Do not lie flat or bend over soon after eating.
- Do not eat late at night or just before bedtime.
- Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some fruits and vegetables.
- Eat slowly and do not eat big meals.
- If you are overweight, lose weight.
- If you smoke, quit smoking.
- Raise the head of your bed.
- Wear loose-fitting clothing around your stomach.
Omeprazole Delayed Release Tablets 20 mg are available in 14 tablet, 28 tablet and 42 tablet sizes. These sizes contain one, two and three 14-day courses of treatment, respectively. Do not use for more than 14 days in a row unless directed by your doctor. For the 28 count (two 14-day courses) and the 42 count (three 14-day courses), you may repeat a 14-day course every 4 months.
For Questions or Comments About Omeprazole Delayed Release Tablets 20 mg
Call 1-800-719-9260
Made in Israel
Manufactured by:
DEXCEL® LTD.
Southern Industrial Zone
Or Akiva 30600, Israel
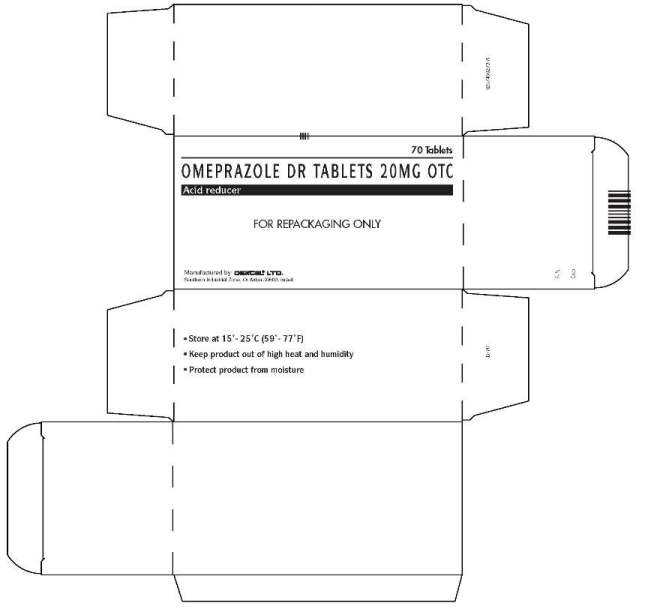
Bulk 7-Count Blister Pack Carton Label
70 Tablets
OMEPRAZOLE DR TABLETS 20 MG OTC
Acid reducer
FOR REPACKAGING ONLY
Manufactured by: DEXCEL® LTD.
Southern Industrial Zone, Or Akiva 30600, Israel
- Store at 15° - 25°C (59° - 77°F)
- Keep product out of high heat and humidity
- Protect product from moisture
| OMEPRAZOLE
DELAYED RELEASE
omeprazole tablet, delayed release |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Dexcel Ltd. (600534200) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dexcel Ltd. | 600534200 | analysis, label, manufacture, pack | |