ADVATE (antihemophilic factor- recombinant kit
ADVATE by
Drug Labeling and Warnings
ADVATE by is a Other medication manufactured, distributed, or labeled by Takeda Pharmaceuticals America, Inc., Baxalta US INC, Baxalta Manufacturing Sarl, Takeda Manufacturing Austria AG, Takeda Manufacturing Singapore Pte. Ltd., Siegfried Hameln GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ADVATE safely and effectively. See full prescribing information for ADVATE.
ADVATE [antihemophilic factor (recombinant)] lyophilized powder for
reconstitution, for intravenous injection
Initial U.S. Approval: 2003INDICATIONS AND USAGE
ADVATE is a recombinant antihemophilic factor indicated for use in children and adults with hemophilia A for:
- Control and prevention of bleeding episodes.
- Perioperative management.
- Routine prophylaxis to prevent or reduce the frequency of bleeding episodes.
ADVATE is not indicated for the treatment of von Willebrand disease. (1)
DOSAGE AND ADMINISTRATION
For intravenous injection after reconstitution only (2)
Each vial of ADVATE contains the labeled amount of recombinant factor VIII in International Units (IU). (2.1)
Control and prevention of bleeding episodes and perioperative management (2.1)
- Dose (IU) = body weight (kg) × desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL).
- Determine treatment frequency based on type of bleeding episode.
Routine Prophylaxis (2.1)
- 20 to 40 IU per kg every other day (3 to 4 times weekly).
- Alternatively, use every third day dosing regimen targeted to maintain FVIII trough levels ≥ 1%.
DOSAGE FORMS AND STRENGTHS
ADVATE is available as a lyophilized powder in single-use vials containing nominally 250, 500, 1000, 1500, 2000, 3000 or 4000 IU. (3)
CONTRAINDICATIONS
Do not use in patients who have life-threatening hypersensitivity reactions, including anaphylaxis, to mouse or hamster protein or other constituents of the product (mannitol, trehalose, sodium chloride, histidine, Tris, calcium chloride, polysorbate 80, and/or glutathione). (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions, including anaphylaxis, may occur. Patients may develop hypersensitivity to mouse or hamster protein, which is present in trace amounts in the product. Should symptoms occur, discontinue treatment with ADVATE and administer appropriate treatment. (5.1)
- Development of activity-neutralizing antibodies may occur. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures factor VIII inhibitor concentration. (5.2, 5.3)
ADVERSE REACTIONS
- Serious adverse drug reactions reported are hypersensitivity and factor VIII inhibitors. (6.1)
- The most common adverse drug reactions observed in greater than 5% of patients are pyrexia, headache, cough, nasopharyngitis, arthralgia, vomiting, upper respiratory tract infection, limb injury, nasal congestion, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Baxalta US Inc. at 1-800-999-1785 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
- Pediatric Use: Clearance (based on per kg body weight) is higher in the pediatric population. Dose adjustment may be needed. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Neutralizing Antibodies
5.3 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ADVATE [Antihemophilic Factor (Recombinant)] is a recombinant antihemophilic factor indicated for use in children and adults with hemophilia A (congenital factor VIII deficiency) for:
- Control and prevention of bleeding episodes.
- Perioperative management.
- Routine prophylaxis to prevent or reduce the frequency of bleeding episodes.
ADVATE is not indicated for the treatment of von Willebrand disease.
-
2 DOSAGE AND ADMINISTRATION
For intravenous injection after reconstitution only.
2.1 Dose
- Dosage and duration of treatment depend on the severity of factor VIII deficiency, the location and extent of the bleeding, and the patient's clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening bleeding episodes.
- Each vial of ADVATE has the recombinant factor VIII potency in International Units (IU) stated on the label. The expected in vivo peak increase in factor VIII level expressed as IU/dL of plasma or percent of normal can be estimated using the following formulas:
IU/dL (or % of normal) = [total dose (IU)/body weight (kg)] × 2 [IU/dL]/[IU/kg]
OR
Required dose (International Units) = body weight (kg) × desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
Examples (assuming patient's baseline factor VIII level is < 1% of normal):
- A dose of 1750 IU ADVATE administered to a 70 kg patient should be expected to result in a peak post-infusion factor VIII increase of: 1750 IU × {[2 IU/dL]/[IU/kg]}/[70 kg] = 50 IU/dL (50% of normal).
- A peak level of 70% is required in a 40 kg child. In this situation, the appropriate dose would be 40 kg × 70 IU/dL/{[2 IU/dL]/[IU/kg]} = 1400 IU.
- Base the dose and frequency on the individual clinical response. Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses to ADVATE. Although the dose can be estimated by the calculations above, whenever possible, perform appropriate laboratory tests including serial factor VIII activity assays. [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)]
Control and Prevention of Bleeding Episodes
A guide for dosing ADVATE for the control and prevention of bleeding episodes is provided in Table 1. The goal of treatment is to maintain a plasma factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in Table 1.
Table 1 Dosing for Control and Prevention of Bleeding Episodes Type of Bleeding Episodes Factor VIII Level Required
(% of normal or IU/dL)Dose*
(IU/kg)Frequency of Doses
(hours)Duration of Therapy
(days)- * Dose (IU/kg) = Desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
Minor
Early hemarthrosis, mild muscle bleeding, or mild oral bleeding episode.20-40 10-20 12-24
(Every 8 to 24 hours for patients under the age of 6)Until the bleeding is resolved (approximately 1 to 3 days). Moderate
Muscle bleeding, bleeding into the oral cavity, definite hemarthroses, and known trauma.30-60 15-30 12-24
(Every 8 to 24 hours for patients under the age of 6)Until the bleeding is resolved (approximately 3 days or more). Major
Significant gastrointestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, central nervous system bleeding, bleeding in the retropharyngeal or retroperitoneal spaces or iliopsoas sheath, fractures, head trauma.60-100 30-50 8-24
(Every 6 to 12 hours for patients under the age of 6)Until bleeding is resolved. Perioperative Management
A guide for dosing ADVATE during surgery (perioperative management) is provided in Table 2. The goal of treatment is to maintain a plasma factor VIII activity level at or above the plasma level (in % of normal or in IU/dL) outlined in Table 2.
Table 2 Dosing for Perioperative Management Type of Surgery Factor VIII Level Required
(% of normal or IU/dL)Dose*
(IU/kg)Frequency of Doses
(hours)Duration of Therapy
(days)- * Dose (IU/kg) = Desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
Minor
Including tooth extraction60-100 30-50 Single dose within one hour of the operation. Single dose or repeat until bleeding is resolved. 12-24 (as needed to control bleeding) For dental procedures, adjunctive therapy may be considered. Major
Intracranial, intra-abdominal, or intrathoracic surgery, joint replacement surgery80-120
(pre- and post-operative)40-60 One dose preoperative to achieve 100% activity.
Every 8-24 to keep factor VIII activity in desired range.
(Every 6 to 24 hours for patients under the age of 6)Until healing is complete. 2.2 Preparation and Reconstitution
Preparation
- Do not remove ADVATE or diluent vials from the external housing.
- Always work on a clean surface and wash your hands before performing the procedures.
- Examine the packaging containing ADVATE to ensure no damage or peeling of the lid is evident. Do not use if the lid is not completely sealed on the blister. Do not remove ADVATE or diluent vials from the external housing.
Reconstitution
- Allow the ADVATE package to reach room temperature.
- Open the package by peeling away the lid. Remove ADVATE from the package and verify that the expiration date on the label has not passed and the potency unit number is same as expected. Inspect parenteral drug products for discoloration and particulate matter. The ADVATE powder should be white to off-white in color and the diluent free from foreign particles. Do not use if the criteria are not met.
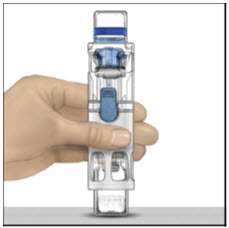
- Place the ADVATE on a flat surface with the diluent vial on top (Figure A). The diluent vial has a blue stripe. Do not remove the blue cap until instructed in a later step.
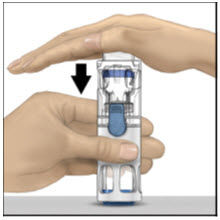
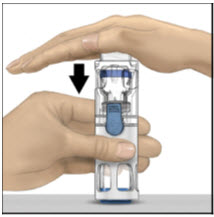
- With one hand holding the ADVATE housing, press down firmly on the diluent vial with the other hand until the system is fully collapsed and the diluent flows down into the ADVATE vial (Figure B). Do not tilt the system until the transfer is complete.
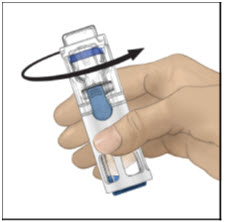
- Verify that diluent transfer is complete. Swirl gently until the powder is completely dissolved (Figure C). Do not shake. Do not refrigerate after reconstitution.
Figure A Figure B Figure C 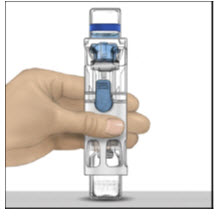
2.3 Administration
For intravenous injection after reconstitution only.
- Inspect parenteral drug products for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance. If not, do not use the solution and notify Baxalta immediately.
- Administer ADVATE at room temperature within 3 hours of reconstitution.
- Use plastic syringes with this product because proteins in the product tend to stick to the surface of glass syringes.
- Use aseptic technique.
- Remove the blue cap from the housing. Connect the syringe to the system (Figure D). Do not inject air into the ADVATE.
- Turn the system upside down (factor concentrate vial now on top). Draw the factor concentrate into the syringe by pulling the plunger back slowly (Figure E).
- Disconnect the syringe, attach a suitable needle, and inject intravenously as instructed. If a patient is to receive more than one ADVATE-BAXJECT III system or a combination of an ADVATE-BAXJECT II and an ADVATE-BAXJECT III system, the contents may be drawn into the same syringe.
- Administer ADVATE over a period of ≤5 minutes (maximum infusion rate 10 mL/min). Determine the pulse rate before and during administration of ADVATE. Should a significant increase in pulse rate occur, reducing the rate of administration or temporarily halting the injection usually allows the symptoms to disappear promptly.
Figure D Figure E 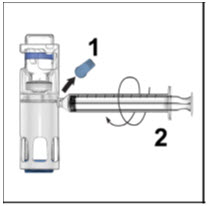
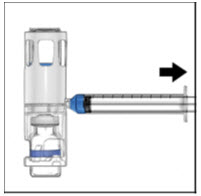
-
3 DOSAGE FORMS AND STRENGTHS
ADVATE is available as a lyophilized white to off-white powder in single-use vials containing nominally 250, 500, 1000, 1500, 2000, 3000, or 4000 International Units (IU, unit). The 250-1500 IU strengths come with 2 mL Sterile Water for Injection (sWFI); the 2000-4000 IU strengths come with 5 mL of sWFI.
Each ADVATE is labeled on the housing with the recombinant antihemophilic factor (rAHF) activity expressed in IU per system. This potency assignment employs a factor VIII concentrate standard that is referenced to a WHO (World Health Organization) international standard for factor VIII concentrates and is evaluated by appropriate methodology to ensure accuracy of the results.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with ADVATE. Symptoms include dizziness, paresthesia, rash, flushing, facial swelling, urticaria, dyspnea, pruritus, and vomiting.
ADVATE contains trace amounts of mouse immunoglobulin G (MuIgG) ≤0.1 ng/IU ADVATE, and hamster proteins ≤1.5 ng/IU ADVATE. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Discontinue ADVATE if hypersensitivity symptoms occur and administer appropriate emergency treatment.
5.2 Neutralizing Antibodies
Neutralizing antibodies (inhibitors) have been reported following administration of ADVATE predominantly in previously untreated patients (PUPs) and previously minimally treated patients (MTPs). Monitor all patients for the development of factor VIII inhibitors by appropriate clinical observation and laboratory testing. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an expected dose, perform an assay that measures factor VIII inhibitor concentration. [see Warnings and Precautions (5.3)]
5.3 Monitoring Laboratory Tests
- Monitor plasma factor VIII activity levels by the one-stage clotting assay to confirm the adequate factor VIII levels have been achieved and maintained when clinically indicated. [see Dosage and Administration (2.1)]
- Monitor for development of factor VIII inhibitors. Perform the Bethesda assay to determine if factor VIII inhibitor is present. If expected factor VIII activity plasma levels are not attained, or if bleeding is not controlled with the expected dose of ADVATE, use Bethesda Units (BU) to titer inhibitors.
- If the inhibitor titer is less than 10 BU per mL, the administration of additional antihemophilic factor concentrate may neutralize the inhibitor and may permit an appropriate hemostatic response.
- If the inhibitor titer is above 10 BU per mL, adequate hemostasis may not be achieved. The inhibitor titer may rise following ADVATE infusion as a result of an anamnestic response to factor VIII. The treatment or prevention of bleeding in such patients requires the use of alternative therapeutic approaches and agents.
-
6 ADVERSE REACTIONS
Serious adverse reactions seen with ADVATE are hypersensitivity reactions, including anaphylaxis, and the development of high-titer inhibitors necessitating alternative treatments to factor VIII.
The most common adverse reactions observed in clinical trials (frequency greater than 5% of subjects) were pyrexia, headache, cough, nasopharyngitis, arthralgia, vomiting, upper respiratory tract infection, limb injury, nasal congestion, and diarrhea.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
ADVATE has been evaluated in eleven clinical trials in previously treated patients (PTPs) and one trial in previously untreated patients (PUPs) with severe to moderately severe hemophilia A (factor VIII ≤2% of normal). A total of 418 subjects have been treated with ADVATE as of January 2012. Total exposure to ADVATE was 63,188 infusions. The median duration of participation per subject was 397 (min-max: 2 −1620) days and the median number of exposure days to ADVATE per subject was 97 (min-max: 1−709).
The summary of adverse reactions with a frequency >5% are shown in Table 3 below.
No subject was withdrawn from a clinical trial due to an adverse reaction.
Table 3 Summary of Adverse Reactions (ARs)* with a Frequency Greater than 5% in 418† Subjects MedDRA‡ System Organ Class MedDRA Preferred Term Number of Adverse Reactions Number of Subjects Percent of Subjects - * Adverse reactions are defined as all adverse events that occurred (a) within 24 hours after being infused with investigational product, or (b) all adverse events assessed related or possibly related to investigational product, or (c) adverse events for which the investigator's or sponsor's opinion of causality was missing or indeterminate.
- † The ADVATE clinical program included 418 treated subjects from 11 completed studies in PTPs and 1 completed trial in PUPs.
- ‡ MedDRA version 8.1 was used.
General Disorders And Administration Site Conditions Pyrexia 110 66 16 Nervous System Disorders Headache 114 56 13 Respiratory, Thoracic And Mediastinal Disorders Cough 86 54 13 Infections And Infestations Nasopharyngitis 72 49 12 Musculoskeletal And Connective Tissue Disorders Arthralgia 49 32 8 Gastrointestinal Disorders Vomiting 41 31 7 Infections And Infestations Upper Respiratory Tract Infection 35 29 7 Injury, Poisoning And Procedural Complications Limb Injury 56 25 6 Respiratory, Thoracic And Mediastinal Disorders Nasal Congestion 32 25 6 Gastrointestinal Disorders Diarrhea 29 24 6 Injury, Poisoning And Procedural Complications Procedural Pain 26 22 5 Respiratory, Thoracic And Mediastinal Disorders Oropharyngeal Pain 25 22 5 Infections And Infestations Ear Infection 30 21 5 Immunogenicity
The development of factor VIII inhibitors with the use of ADVATE was evaluated in clinical trials with pediatric PTPs (<6 years of age with ≥50 factor VIII exposures) and PTPs (≥10 years of age with ≥150 factor VIII exposures). Of 276 subjects who were treated with ADVATE for at least 10 exposure days or on study for a minimum of 120 days, 1 adult developed a low-titer inhibitor (2 BU in the Bethesda assay) after 26 exposure days. Eight weeks later, the inhibitor was no longer detectable, and in vivo recovery was normal at 1 and 3 hours after infusion of another marketed recombinant factor VIII concentrate. This event results in a factor VIII inhibitor frequency in PTPs of 0.4% (95% CI of 0.01 and 2% for the risk of any factor VIII inhibitor development).3 No factor VIII inhibitors were detected in the 53 treated pediatric PTPs.
In clinical trials that enrolled previously untreated subjects (defined as having had up to 3 exposures to a factor VIII product at the time of enrollment) , 16 (29.1%) of 55 subjects who received ADVATE developed inhibitors to factor VIII. Seven subjects developed high titer (>5 BU) and nine subjects developed low-titer inhibitors. Inhibitors were detected at a median of 13 exposure days (min-max: 6 −26 exposure days) to the product.
Immunogenicity also was evaluated by measuring the development of antibodies to heterologous proteins. When assessed for anti-Chinese hamster ovary (CHO) cell protein antibodies, of 229 treated subjects, 3 showed an upward trend in antibody titer over time and 10 showed repeated but transient elevations of antibodies. When assessed for muIgG protein antibodies, of the 229 treated subjects, 10 showed an upward trend in anti-muIgG antibody titer over time and 2 showed repeated but transient elevations of antibodies. Four subjects who demonstrated antibody elevations to CHO cell or muIgG proteins, reported isolated events of urticaria, pruritus, rash, and slightly elevated eosinophil counts. All of these subjects had numerous repeat exposures to the product without recurrence of the events and a causal relationship between the antibody findings and these clinical events has not been established.
When assessed for the presence of anti-human von Willebrand Factor (VWF) antibodies, of the 228 treated subjects, none displayed laboratory evidence indicative of a positive serologic response.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to ADVATE with the incidence of antibodies to other products may be misleading.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ADVATE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Table 4 represents the most frequently reported post-marketing adverse reactions as MedDRA Preferred Terms.
Table 4 Post-Marketing Experience Organ System [MedDRA Primary SOC] Preferred Term Immune system disorders Anaphylactic reaction
HypersensitivityGeneral disorders and administration site conditions Injection site reaction
Chills
Fatigue/Malaise
Chest discomfort/pain
Decreased therapeutic effect -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with ADVATE use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with ADVATE. It is not known whether ADVATE can cause fetal harm when administered to a pregnant woman or whether it can affect reproductive capacity.
In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ADVATE in human milk, the effect on the breastfed infant, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ADVATE and any potential adverse effects on the breastfed child from ADVATE or from the underlying maternal condition.
8.4 Pediatric Use
Pharmacokinetic studies in children have demonstrated higher clearance, a shorter half-life and lower recovery of factor VIII compared to adults. [see Clinical Pharmacology (12.3)] This may be explained by differences in body composition and should be taken into account when dosing or following factor VIII levels in the pediatric population.4 Because clearance (based on per kg body weight) has been demonstrated to be higher in the pediatric population, dose adjustment or more frequent dosing based on per kg body weight may be needed in this population. [see Clinical Pharmacology (12.3)] In the ADVATE routine prophylaxis clinical trial, 3 children aged 7 to <12 and 4 adolescents aged 12 to < 16 were included in the per-protocol analysis. The reductions in annualized bleeding rate per subject per year during any prophylaxis regimen as compared to during on-demand therapy were similar among children, adolescents, and adults. [see Clinical Studies (14)]
8.5 Geriatric Use
Clinical trials of ADVATE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease and other drug therapy.
-
11 DESCRIPTION
ADVATE [Antihemophilic Factor (Recombinant)] is a purified glycoprotein consisting of 2,332 amino acids that is synthesized by a genetically engineered Chinese hamster ovary (CHO) cell line but does not contain plasma or albumin. The CHO cell line employed in the production of ADVATE is derived from that used in the biosynthesis of RECOMBINATE [Antihemophilic Factor (Recombinant)]. ADVATE has been shown to be comparable to RECOMBINATE with respect to its biochemical and physicochemical properties, as well as its non-clinical in vivo pharmacology.
In culture, the CHO cell line expresses the recombinant antihemophilic factor (rAHF) into the cell culture medium. The rAHF is purified from the culture medium using a series of chromatography columns. The purification process includes an immunoaffinity chromatography step in which a monoclonal antibody directed against factor VIII is employed to selectively isolate the rAHF from the medium. The cell culture and purification processes used in the manufacture of ADVATE employ no additives of human or animal origin. The production process includes a dedicated, viral inactivation solvent-detergent treatment step. The rAHF synthesized by the CHO cells has the same biological effects on clotting as human antihemophilic factor (hAHF). Structurally the recombinant protein has a similar combination of heterogeneous heavy and light chains as found in AHF (Human).
ADVATE is formulated as a sterile, non-pyrogenic, white to off-white powder for intravenous injection. ADVATE in a single-use vial contains nominally 250, 500, 1000, 1500, 2000, 3000, or 4000 International Units (IU). The product contains the following stabilizers and excipients: mannitol, trehalose, sodium chloride, histidine, Tris, calcium chloride, polysorbate 80, and glutathione. Von Willebrand factor (VWF) is co-expressed with factor VIII and helps to stabilize it in culture. The final product contains no more than 2 ng VWF/IU rAHF, which will not have any clinically relevant effect in patients with von Willebrand disease.
The product contains no preservative. When reconstituted with the provided Sterile Water for Injection, USP, the final solution contains the following stabilizers and excipients in targeted amounts:
Table 5 Approximate Concentration of Stabilizer and Excipient after Reconstitution Stabilizer and Excipient 2 mL Reconstitution
(for 250, 500, 1000, 1500 IU)
Target5 mL Reconstitution
(for 2000, 3000, 4000 IU)
TargetTris (hydroxymethyl) aminomethane 25 mM 10 mM Calcium Chloride 4.2 mM 1.7 mM Mannitol 8 % (w/v) 3.2% (w/v) Sodium Chloride 225 mM 90mM α, α-Trehalose 2% (w/v) 0.8% (w/v) Histidine 25 mM 10 mM Glutathione (Reduced) 0.2 mg/mL 0.08 mg/mL Polysorbate 80 0.025% (w/v) 0.01% (w/v) Each ADVATE housing is labeled with the rAHF activity expressed in international units. Biological potency is determined by an in vitro assay, which employs a factor VIII concentrate standard that is referenced to a WHO international standard for factor VIII concentrates. One international unit, as defined by the WHO standard for blood coagulation factor VIII, human, is approximately equal to the level of factor VIII activity found in 1 mL of fresh pooled human plasma. The specific activity of ADVATE is 4000 to 10000 International Units per milligram of protein.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ADVATE temporarily replaces the missing coagulation factor VIII that is needed for effective hemostasis.
12.2 Pharmacodynamics
The activated partial thromboplastin time (aPTT) is prolonged in patients with hemophilia. Determination of aPTT is a conventional in vitro assay for biological activity of factor VIII. Treatment with ADVATE normalizes the aPTT over the effective dosing period.
12.3 Pharmacokinetics
A randomized, crossover pharmacokinetic trial of ADVATE (test) and RECOMBINATE [Antihemophilic Factor (Recombinant)] (reference) was conducted in 56 non-bleeding subjects. The subjects received either of the products as an IV infusion (50 ± 5 IU/kg body weight) and there was a washout period of 72 hours to 4 weeks between the two infusions. The pharmacokinetic parameters were calculated from factor VIII activity measurements in blood samples obtained up to 48 hours following each infusion.3 The per-protocol analysis included 30 patients (20 adults and 10 children). Pharmacokinetic parameters for the 20 adults for each trial are presented in Table 6.
Table 6 Pharmacokinetic Parameters (Mean ± SD) for ADVATE and RECOMBINATE (N=20 Adult Subjects Age >16 years) Parameter RECOMBINATE
(n=20)ADVATE
(n=20)- * Area under the plasma factor VIII concentration × time curve from 0 to 48 hours post-infusion.
- † Calculated as (Cmax – baseline factor VIII) divided by the dose in IU/kg, where Cmax is the maximal post-infusion factor VIII measurement.
AUC0-48h (IU∙hrs/dL)* 1638 ± 357 1644 ± 338 In vivo recovery (IU/dL/IU/kg)† 2.7 ± 0.6 2.6 ± 0.5 Half-life (hrs) 11.2 ± 2.5 12.0 ± 4.2 Cmax (IU/dL) 136 ± 29 128 ± 28 MRT (hrs) 14.7 ± 3.8 15.8 ± 5.9 Vss (dL/kg) 0.4 ± 0.1 0.4 ± 0.1 CL (mL/kg*hr) 3 ± 1 3 ± 1 The 90% confidence intervals for the ratios of the mean AUC(0-48h) and in vivo recovery values for the test and control products were within the pre-established limits of 0.80 and 1.25. In addition, in vivo recoveries at the onset of treatment and after 75 exposure days were compared for 62 subjects. Results of this analysis indicated no significant change in the in vivo recovery at the onset of treatment and after ≥ 75 exposure days.
In an analysis of data from 58 subjects with 65 surgical procedures in the perioperative management trial, the target factor VIII level was met or exceeded in all cases following a single loading dose ranging from 29 to 104 IU/kg.
Pharmacokinetic parameters calculated from 98 subjects less than 16 years of age (intent-to-treat analysis) are available for 7 infants (1 month to less than 2 years), 32 children (2 to less than 5 years), 24 older children (5 to less than 12 years), and 35 adolescents (12 to less than 16 years), as shown in Table 7. The mean clearance (based on body weight) of ADVATE in infants, children, older children, and adolescents was higher than adults (3.6 mL/kg*hr). The mean half-life of ADVATE in infants, children, older children, and adolescents was lower than adults (12 hours). The extent to which these differences may be clinically significant is not known.
Table 7 Pharmacokinetic Parameters (Mean ± SD) of ADVATE by Age Group < 16 Years (N=98; Intent-to-Treat PK Analysis Set) PK Parameter Infants
(N=7)
(1 month to <2 yrs)Children
(N=32)
(2 to <5 yrs)Older Children
(N=24)
(5 to < 12 yrs)Adolescents
(N=35)
(12 to < 16 years)- * Incremental recovery at Cmax calculated as (Cmax – baseline factor VIII) divided by the dose in IU/kg, where Cmax is the maximal post-infusion factor VIII measurement
AUC0-inf (IU*hr/dL) 1240 ± 330 1164 ± 424 1396 ± 506 1300 ± 469 Incremental Recovery at Cmax * (IU/dL per IU/kg) 2.1 ± 0.5 1.8 ± 0.4 2.1 ± 0.6 2.1 ± 0.5 Half-Life (hr) 8.7 ± 1.4 9.5 ± 1.8 11.2 ± 3.5 12.0 ± 2.9 Maximum Plasma Concentration Post Infusion (IU/dL) 104 ± 27 91 ± 19 105 ± 34 103 ± 25 Mean Residence Time (hr) 10.4 ± 2.5 11.8 ± 2.8 14.3 ± 4.3 14.9 ± 4.6 Volume of Distribution at Steady State (dL/kg) 0.4 ± 0.1 0.5 ± 0.1 0.6 ± 0.2 0.6 ± 0.1 Clearance (mL/kg*hr) 4.3 ± 1.0 4.8 ± 1.5 4.1 ± 1.5 4.2 ± 1.2 In a crossover pharmacokinetic trial of recombinant antihemophilic factor, plasma/albumin free method (rAHF-PFM) reconstituted in 2 mL versus 5 mL Sterile Water for Injection, USP (sWFI) in previously treated severe hemophilia A adult and adolescent patients, the AUCs of the two formulations were comparable and the 90% confidence interval ranged from 90.4 to 102.6, indicating that the two formulations are pharmacokinetically equivalent.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted with the active ingredient in ADVATE to assess its mutagenic or carcinogenic potential.
RECOMBINATE was tested for mutagenicity at doses considerably exceeding plasma concentrations in vitro, and at doses up to ten times the expected maximal clinical dose in vivo. At that concentration, it did not cause reverse mutations, chromosomal aberrations, or an increase in micronuclei formation in bone marrow polychromatic erythrocytes. Studies in animals have not been performed to evaluate carcinogenic potential.
-
14 CLINICAL STUDIES
Original Safety and Efficacy Study
A safety and efficacy trial evaluated the pharmacokinetics (double-blinded, randomized, cross-over), safety, immunogenicity, and hemostatic efficacy (open-label) of ADVATE in 111 subjects. The trial was conducted in the US and Europe with 103 Caucasian, 7 Black, and 1 Asian previously treated subjects (PTPs with ≥ 150 exposure days) diagnosed with moderate to severe hemophilia A (FVIII level ≤ 2% of normal) who were ≥10 years of age (20 were 10 to <13, 22 were 13 to <16, and 69 were 16 years and older). Subjects with a history of, or a detectable FVIII inhibitor were excluded.
Subjects self-administered ADVATE for routine prophylaxis (≥25 IU/kg body weight 3-4 times per week) and for the on-demand treatment of bleeding episodes. A global assessment of efficacy was rendered by the subject (for home treatment) or study site investigator (for treatment under medical supervision) using a scale of excellent, good, fair, or none, based on the quality of hemostasis achieved with ADVATE for the treatment of each new bleeding episode.
A total of 510 bleeding episodes were reported, with a mean (± SD) of 6.1 ± 8.2 bleeding episodes per subject. Of these 510 episodes, 439 (86%) were rated excellent or good in their response to treatment with ADVATE, 61 (12%) were rated fair, 1 (0.2%) was rated as having no response, and for 9 (2%), the response to treatment was unknown. A total of 411 (81%) bleeding episodes were managed with a single infusion, 62 (12%) required 2 infusions, 15 (3%) required 3 infusions, and 22 (4%) required 4 or more infusions of ADVATE for satisfactory resolution. A total of 162 (32%) bleeding episodes occurred spontaneously, 228 (45%) were the result of antecedent trauma, and for 120 (24%) bleeding episodes, the etiology was unknown.3
The rate of new bleeding episodes during the 75-exposure-day prophylactic regimen was calculated as a function of the etiology of bleeding episodes for 107 evaluable subjects (n = 274 bleeding episodes).3 These rates are presented in Table 8. The overall rate of new bleeding episodes in the prophylaxis study was 0.52 ± 0.71.
Table 8 Rate of New Bleeding Episodes During Prophylaxis Types of Bleeding Episode Mean (± SD) New Bleeding Episodes/Subject/Month Spontaneous 0.34 ± 0.49 Post-traumatic 0.39 ± 0.46 Unknown/Indeterminate 0.33 ± 0.34 The pharmacokinetic properties of ADVATE were investigated at the beginning of treatment in a multicenter trial of previously treated subjects and at the end of treatment in a subset of subjects (N=34) who had completed at least 75 exposure days of treatment with ADVATE. [Clinical Pharmacology (12.3)]
Continuation Study
Additional (open-label) safety and efficacy data were collected on 82 subjects who continued with treatment following participation in the original safety and efficacy study. Bleeding episodes were treated with ADVATE and the outcome of treatment was rated as excellent, good, fair, or none, based on the quality of hemostasis achieved. Final analysis of efficacy was conducted for 81 subjects who self-administered ADVATE on a routine prophylactic regimen for a minimum period of 75 exposure days.
A total of 837 bleeding episodes occurred in 70 of the 81 subjects. The other 11 subjects experienced no bleeding episodes. The response to treatment with ADVATE was rated as excellent or good for 80.4% of all bleeding episodes. Most (88%) bleeding episodes required only 1 or 2 infusions to obtain hemostasis. Among the 837 bleeding episodes, 2 (0.3%) did not require treatment (0 infusions), 521 (62.2%) required 1 infusion, 216 (25.8%) required 2 infusions, 23 (2.7%) required 3 infusions, and 75 (9.0%) required 4 or more infusions. By etiology, 45.3% of these bleeding events were secondary to trauma and 27.7% occurred spontaneously; the other 27% had an undetermined etiology.
In vivo recoveries at the onset of treatment and after 75 exposure days were compared for 62 subjects and there were no significant differences.
Perioperative Management Study
The safety and efficacy of ADVATE for perioperative management was investigated in 59 subjects with severe or moderately severe hemophilia A (factor VIII ≤ 2%). They were between the ages of 7 to 65 years of age (3 were 7 to <13, 6 were 13 to < 16, and 50 were ≥ 16). Fifty-five were Caucasian, 3 were Black, and 1 was Asian. One subject elected not to undergo the planned surgery. Thus, 58 subjects underwent 65 surgical procedures, among which, 6 subjects underwent more than 1 procedure each. One subject withdrew during the postoperative period; thus, 57 subjects completed the study. Of the 65 procedures, 22 in 22 subjects were classified as major, 35 in 28 subjects were classified as minor, and 8 in 8 subjects were dental. (See Table 2 for definitions of major and minor)
Prior to surgery, subjects received a pre-operative loading dose aimed at increasing the plasma factor VIII level to 60% to 100% of normal for dental procedures or 80% to 120% of normal for all other surgical procedures. During the surgery, subjects received replacement therapy by either bolus (47 procedures) or continuous infusion (18 procedures). For continuous infusion, the initial rate was 4 IU/kg/hr for subjects >12 years of age and 5 IU/kg/hr for subjects 5 to 12 years of age. After discharge, subjects continued to receive ADVATE for control of hemostasis as prescribed by the investigator for up to 6 weeks for major orthopedic procedures and up to 2 weeks for all other procedures.
Intraoperative efficacy was rated as excellent or good (Excellent intraoperative blood loss was less than expected for the type of procedure performed; Good intraoperative blood loss was as expected for the type of procedure performed.) for 61 (93.9%) of the 65 procedures; the rating was not done for 3 procedures and unknown for 1 procedure. Postoperative efficacy was rated as excellent or good for 62 (95.4%) of the 65 procedures; the rating was unknown for 2 procedures and not done for 1 procedure. Of the 24 procedures requiring surgical drains, efficacy assessments at the time of drain removal were rated as excellent or good for 20 (83.3%) procedures and fair (Fair intraoperative blood loss was more than expected for the type of procedure performed) for 2 (8.3%) procedures; the rating was unknown for 1 procedure and not done for 1 procedure. Both procedures requiring surgical drains with fair ratings were major orthopedic surgeries.
Routine Prophylaxis Study
In a multicenter, open-label, prospective, randomized, controlled postmarketing clinical trial of ADVATE use in two prophylactic treatment regimens compared to that of on-demand treatment, 53 PTPs with severe to moderately severe hemophilia A (FVIII level ≤ 2 IU/dL) were analyzed in the per-protocol group. Subjects were initially treated for 6 months of on-demand therapy and then randomized to 12 months of either a standard prophylaxis regimen (20-40 IU/kg every 48 hours) or PK-driven prophylaxis regimen (20-80 IU/kg every 72 hours). All subjects had a history of at least 8 joint bleeding episodes per year upon entering the trial. Each subject in the per-protocol group was adherent to > 90% of the prescribed number of prophylactic infusions; no subject in the trial surpassed the upper boundary of 110% of the prescribed number of prophylactic infusions.
The equation used to determine the weight-adjusted dose of the product used in the PK-driven prophylaxis arm, as calculated from the individual subject's incremental recovery and half-life values to achieve a trough level of ≥ 1 IU/dL at the inter-dosing interval of 72 hours is defined as follows:
- Di = (2)72/ti / ri (i is the subject)
-
D = target FVIII dose (IU/kg) that ensures that a trough level of ≥1 IU/dL is achieved after 72 hours
r = FVIII incremental recovery (IU/dL / IU/kg) as determined by the subject's PK analysis
t = FVIII half-life (hrs) as determined by the subject's PK analysis
The median annual bleed rate during the on-demand therapy period was 44 bleeds per subject per year compared to 1 bleed per subject per year while on either prophylaxis regimen, which was a statistically significant difference (p<0.0001). Twenty-two of 53 (42%) subjects experienced no bleeding episodes while on prophylaxis for one year. While there was no statistically significant difference in bleeding frequency observed between the two prophylaxis regimens studied, the trial was not powered to demonstrate equivalence in bleeding rate between the two prophylaxis arms.
Table 9 Annual Bleed Rate of Prophylaxis Compared to On-Demand Treatment Clinical Parameters On-Demand
(n=53)Standard Prophylaxis
(n=30)PK-Driven Prophylaxis
(n=23)Either Standard or PK-Driven Prophylaxis
(n=53)- * Inter-quartile-range (IQR) is defined as the difference between the 75th percentile (3rd quartile) and the 25th percentile (first quartile).
Median (IQR)* Annual Bleed Rate(ABR) 44.0 (20.8) 1.0 (2.1) 1.0 (4.1) 1.0 (4.1) Median (IQR) * Joint ABR 38.7 (24.8) 0.5 (2.0) 1.0 (4.1) 1.0 (2.1) Median (IQR) * Non-Joint ABR1 4.0 (11.9) 0.0 (0.0) 0.0 (0.0) 0.0 (0.0) Median (IQR)* Spontaneous ABR 32.0 (26.8) 0.0 (1.9) 0.0 (2.0) 0.0 (1.9) Median (IQR)* Traumatic ABR 11.5 (17.2) 0.0 (1.0) 1.0 (1.0) 0.0 (1.0) The annualized bleed rates by age category during on-demand and either standard or PK-driven prophylaxis regimens are shown in Table 10.
Table 10 Annualized Bleed Rate by Age Category and Any Prophylaxis vs On-Demand (Per Protocol) Any Prophylaxis On-Demand Age Category Number of Subjects Median Percentage of Subjects With Zero Bleeds Median Percentage of Subjects With Zero Bleeds Children
(≥7 to <12 years old)3 5.2 33% 44.0 All subjects bleed during On-Demand Adolescents
(≥12 to <16 years old)4 5.0 25% 58.0 Adults
(≥16 years old and older)46 1.0 43% 44.7 All Subjects 53 1.0 42% 44.0 As a secondary endpoint, the trial assessed all Short Form Health Survey (SF-36v1) domains. The SF-36v1 is a valid and reliable measure of health-related quality of life that is comprised of 8 domains categorized into 2 summary scores (Table 11).
Table 11 Mean Change in SF-36v1 Health Domain Scores Between End of On-Demand and End of Prophylaxis Treatment Regimens* SF-36v1 Health Domain Mean Change 95% Confidence Interval - * Positive change values are in the favorable direction.
Physical Functioning (PF) 0.89 (-1.02, 2.81) Role Physical (RP) 3.56 (0.32, 6.79) Bodily Pain (BP) 4.13 (1.63, 6.62) General Health (GH) 1.36 (-0.72, 3.45) Physical Component Score 3.56 (1.56, 5.56) Vitality (VT) 0.21 (-2.22, 2.63) Social Functioning (SF) 1.72 (-0.57, 4.00) Role Emotional (RE) -1.29 (-3.78, 1.19) Mental Health (MH) -0.20 (-2.89, 2.49) Mental Component Score -1.22 (-3.66, 1.23) Human Factors Usability Study
A human factors study was performed with 44 participants to evaluate the usability of the ADVATE in the BAXJECT III reconstitution system. Participants in the study included 15 patients, 16 caregivers, and 13 healthcare providers.
During the study, participants viewed an instructional video then performed the reconstitution steps utilizing the instructions for use (IFU). Objective performance data were collected and evaluated. Participants' comments from a post-evaluation interview were reviewed for their appropriateness and applicability. As a result, the content of the package insert was revised to clarify the instructions for use.
-
15 REFERENCES
- Fischer K, Collins P, Björkman S, Blanchette V, Oh M, Fritsch S, Schroth P, Spotts G, Ewenstein B. Trends in bleeding patterns during prophylaxis for severe haemophilia: observations from a series of prospective clinical trials. Haemophilia 2011 17(3):433-8.
- Collins PW, Blanchette VS, Fischer K, Björkman S, Oh M, Fritsch S, Schroth P, Astermark J, Spotts G, Ewenstein B, The rAHF-PFM Study Group. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe haemophilia A. J Thromb Haemost 2009 7(3):413-20.
- Tarantino MD, Collins PW, Hay PW et al. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia 2004 10:428-437.
- Björkman S, Blanchette VS, Fischer K, Oh M, Spotts G, Schroth P, Fritsch S, Patrone L, Ewenstein BM, Collins PW, ADVATE Clinical Program Group. Comparative pharmacokinetics of plasma- and albumin-free recombinant factor VIII in children and adults: the influence of blood sampling schedule on observed age-related differences and implications for dose tailoring. J Thromb Haemost 2010 8(4):730-6.
- Negrier C, Shapiro A, Berntorp E et al. Surgical evaluation of a recombinant factor VIII prepared using a plasma/albumin-free method: efficacy and safety of ADVATE in previously treated patients. Thromb.Haemost 2008100:217-223.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ADVATE in a BAXJECT III system is packaged with 2 mL or 5 mL of Sterile Water for Injection, one Terumo Microbore Infusion set (2 mL only), one full prescribing physician insert, and one patient insert.
ADVATE is available in single-dose vials that contain the following nominal product strengths:
Nominal Strength Factor VIII Potency Range Potency Color Code Carton NDC
(Includes 2 mL sWFI Diluent)Carton NDC
(Includes 5 mL sWFI Diluent)250 IU 200-400 IU per vial Light blue 0944-3051-02 500 IU 401-800 IU per vial Pink 0944-3052-02 1000 IU 801-1200 IU per vial Green 0944-3053-02 1500 IU 1201-1800 IU per vial Purple 0944-3054-02 2000 IU 1801-2400 IU per vial Orange 0944-3045-10 3000 IU 2401-3600 IU per vial Silver 0944-3046-10 4000 IU 3601- 4800 IU per vial Dark Green 0944-3047-10 Actual factor VIII activity in International Units is stated on the label of each ADVATE housing or carton.
Not made with natural rubber latex.
Storage and Handling
- Refrigerate ADVATE in powder form at 2° - 8°C (36° - 46°F).
- Store at room temperature up to 30°C (86°F) for a period of up to 6 months not to exceed the expiration date.
- Record on the carton the date ADVATE is removed from refrigeration. The product must not be returned to refrigerated temperature.
- Do not use beyond the expiration date printed on the ADVATE label or carton.
- Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Advise patients to report any adverse reactions or problems following ADVATE administration to their physician or healthcare provider.
- Allergic-type hypersensitivity reactions have been reported with ADVATE. Warn patients of the early signs of hypersensitivity reactions, including hives, pruritus, generalized urticaria, angioedema, hypotension, shock, anaphylaxis and acute respiratory distress. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment.
- Inhibitor formation may occur with the treatment of a patient with hemophilia A. Advise patients to contact their physician or treatment center if they experience a lack of clinical response to factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
- Advise patients to consult with their physicians or healthcare provider prior to travel. While traveling, advise patients to bring an adequate supply of ADVATE based on their current regimen of treatment.
To enroll in the confidential, industry-wide Patient Notification System, call 1-888-873-2838.
-
SPL UNCLASSIFIED SECTION
BAXALTA®, ADVATE®, BAXJECT® and RECOMBINATE® are trademarks of Baxalta Incorporated, a wholly-owned, indirect subsidiary of Shire plc.
SHIRE and the Shire Logo are trademarks or registered trademarks of Shire Pharmaceutical Holdings Ireland Limited or its affiliates.
Patented: see https://www.shire.com/legal-notice/product-patents
Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020 -
FDA-Approved Patient Labeling
ADVATE (ad-vate)
[Antihemophilic Factor (Recombinant)]This leaflet summarizes important information about ADVATE. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about ADVATE. If you have any questions after reading this, ask your healthcare provider.
What is the most important information I need to know about ADVATE?
Do not attempt to do an infusion to yourself unless you have been taught how by your healthcare provider or hemophilia center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for infusing ADVATE so that your treatment will work best for you.
What is ADVATE?
ADVATE is a medicine used to replace clotting factor (factor VIII or antihemophilic factor) that is missing in people with hemophilia A (also called "classic" hemophilia). The product does not contain plasma or albumin. Hemophilia A is an inherited bleeding disorder that prevents blood from clotting normally.
ADVATE is used to prevent and control bleeding in adults and children (0-16 years) with hemophilia A.
Your healthcare provider may give you ADVATE when you have surgery.
ADVATE can reduce the number of bleeding episodes in adults and children (0-16 years) when used regularly (prophylaxis).
ADVATE is not used to treat von Willebrand disease.
Who should not use ADVATE?
You should not use ADVATE if you:
- Are allergic to mice or hamsters.
- Are allergic to any ingredients in ADVATE.
Tell your healthcare provider if you are pregnant or breastfeeding because ADVATE may not be right for you.
How should I use ADVATE?
ADVATE is given directly into the bloodstream.
You may infuse ADVATE at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your healthcare provider or hemophilia treatment center. Many people with hemophilia A learn to infuse their ADVATE by themselves or with the help of a family member.
Your healthcare provider will tell you how much ADVATE to use based on your weight, the severity of your hemophilia A, and where you are bleeding.
You may have to have blood tests done after getting ADVATE to be sure that your blood level of factor VIII is high enough to clot your blood.
Call your healthcare provider right away if your bleeding does not stop after taking ADVATE.
What should I tell my healthcare provider before I use ADVATE?
You should tell your healthcare provider if you:
- Have or have had any medical problems.
- Take any medicines, including prescription and non-prescription medicines, such as over-the-counter medicines, supplements or herbal remedies.
- Have any allergies, including allergies to mice or hamsters.
- Are breastfeeding. It is not known if ADVATE passes into your milk and if it can harm your baby.
- Are pregnant or planning to become pregnant. It is not known if ADVATE may harm your unborn baby.
- Have been told that you have inhibitors to factor VIII (because ADVATE may not work for you).
What are the possible side effects of ADVATE?
You can have an allergic reaction to ADVATE.
Your body may form inhibitors to factor VIII. An inhibitor is part of the body's normal defense system. If you form inhibitors, it may stop ADVATE from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to factor VIII.
Call your healthcare provider right away and stop treatment if you get a rash or hives, itching, tightness of the throat, chest pain or tightness, difficulty breathing, lightheadedness, dizziness, nausea or fainting.
Side effects that have been reported with ADVATE include:
cough
sore throat
unusual taste
abdominal pain
diarrhea
nausea/vomitingheadache
fever
dizziness
hot flashes
chills
sweatingjoint swelling/aching
itching
hematoma
swelling of legs
runny nose/congestion
rashTell your healthcare provider about any side effects that bother you or do not go away.
These are not all the possible side effects with ADVATE. You can ask your healthcare provider for information that is written for healthcare professionals.
What are the ADVATE dosage strengths?
ADVATE with 2 mL or 5 mL Sterile Water for Injection in a BAXJECT III system comes in six different dosage strengths: 250 International Units (IU), 500 IU, 1000 IU, 1500 IU, 2000 IU, 3000 IU and 4000 IU. The actual strength will be imprinted on the label on the housing and on the box. The six different strengths are color coded, as follows:

Dosage strength of approximately 250 International Units (200 – 400 IU) (with 2 mL sWFI) 
Dosage strength of approximately 500 International Units (401 – 800 IU) (with 2 mL sWFI) 
Dosage strength of approximately 1000 International Units (801 – 1200 IU) (with 2 mL sWFI) 
Dosage strength of approximately 1500 International Units (1201 – 1800 IU) (with 2 mL sWFI) 
Dosage strength of approximately 2000 International Units (1801 – 2400 IU) (with 5 mL sWFI) 
Dosage strength of approximately 3000 International Units (2401 – 3600 IU) (with 5 mL sWFI) 
Dosage strength of approximately 4000 International Units (3601 – 4800 IU) (with 5 mL sWFI) Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider. Always check the expiration date printed on the box. Do not use the product after the expiration date printed on the box.
How do I store ADVATE?
Do not freeze ADVATE.
Store ADVATE in a refrigerator (2° to 8°C [36° to 46°F]) or at room temperature (up to 30°C [86°F]) for up to 6 months.
If you choose to store ADVATE at room temperature:
- Note the date that the product is removed from refrigeration on the box.
- Do not use after six months from this date or after the expiration date.
- Do not return the product back to the refrigerator.
Store ADVATE in the original box and protect from extreme exposure to light.
Reconstituted product (after mixing dry product with wet diluent) must be used within 3 hours and cannot be stored or refrigerated. Discard any unused ADVATE at the end of your infusion.
What else should I know about ADVATE and Hemophilia A?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use ADVATE for a condition for which it is not prescribed. Do not share ADVATE with other people, even if they have the same symptoms that you have.
Resources at Baxalta available to the patients:
For information on patient assistance programs that are available to you, including the Baxalta CARE Program, please contact the Baxalta Insurance Assistance Helpline at 1-888-229-8379.
Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020Issued: 12/2018
-
INSTRUCTIONS FOR USE
ADVATE
[Antihemophilic Factor (Recombinant)]
(For intravenous use only)Do not attempt to do an infusion to yourself unless you have been taught how by your healthcare provider or hemophilia center.
See below for step-by-step instructions for reconstituting ADVATE in a BAXJECT III system.
- Always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using ADVATE. If you are unsure of the procedures, please call your healthcare provider before using.
- Call your healthcare provider right away if bleeding is not controlled after using ADVATE.
- Your healthcare provider will prescribe the dose that you should take.
- Your healthcare provider may need to take blood tests from time to time.
- Talk to your healthcare provider before traveling. Plan to bring enough ADVATE for your treatment during this time.
- Dispose of all materials, including any leftover reconstituted ADVATE product, in an appropriate container.
- 1. Prepare a clean flat surface and gather all the materials you will need for the infusion. Check the expiration date, and let the ADVATE warm up to room temperature. Wash your hands and put on clean exam gloves. If infusing yourself at home, the use of gloves is optional.
- 2. Open the ADVATE package by peeling away the lid. Remove the ADVATE from the package and visually inspect the contents of the product and diluent vial. The ADVATE powder should be white to off-white in color and the diluent should not contain particles. Do not use if discoloration or particles are seen.
- 3.
Place on a flat surface with the diluent vial on top. The diluent vial has a blue stripe.
- 4.
With one hand holding the ADVATE housing, press down firmly on the diluent vial with the other hand until the system is fully collapsed and the diluent flows down into the ADVATE vial. Both vials will move into the housing when pressed. If you don't see the diluent transfer to the product vial, press the vials again to assure they are completely inserted. Do not remove the blue cap until instructed in a later step.
- 5.
Swirl the ADVATE gently and continuously until the ADVATE is completely dissolved. Do not shake. Do not refrigerate after reconstitution. Inspect the ADVATE solution for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance. If not, do not use the solution and notify your healthcare provider immediately.
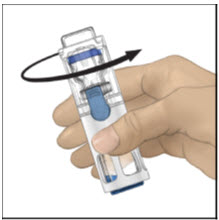
- 6.
Take off the blue cap from the housing and connect the syringe. Be careful to not inject air into the ADVATE.
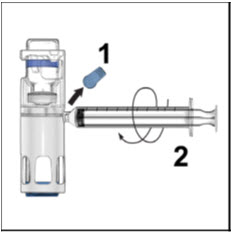
- 7.
Turn over the ADVATE so that the vial containing the ADVATE solution is on top. Draw the ADVATE solution into the syringe by pulling back the plunger slowly. If the solution does not draw into the syringe, be sure that both vials are pressed firmly together. The contents of more than one vial may be drawn into a single, appropriately sized syringe if you are using more than one vial of ADVATE.
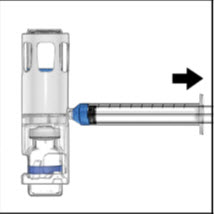
- 8. Disconnect the syringe from the system. Attach the infusion needle to the syringe using a winged (butterfly) infusion set, if available. Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe and needle.
- 9.
Apply a tourniquet and get the injection site ready by wiping the skin well with an alcohol swab (or other suitable solution suggested by your healthcare provider or hemophilia center).
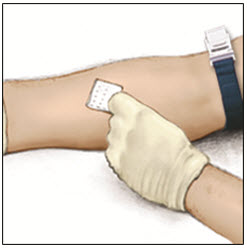
- 10.
Insert the needle into the vein and remove the tourniquet. Slowly infuse the ADVATE. Do not infuse any faster than 10 mL per minute.
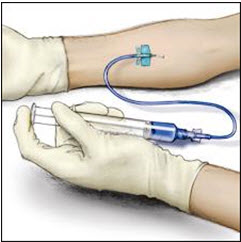
- 11. Take the needle out of the vein and use sterile gauze to put pressure on the infusion site for several minutes.
- 12. Do not recap the needle. Place the needle, syringe, and ADVATE in a hard-walled sharps container for proper disposal. Do not dispose of these supplies in ordinary household trash.
- 13. Remove the peel-off label from the housing and place it in your logbook. Clean any spilled blood with a freshly prepared mixture of 1 part bleach and 9 parts water, soap and water, or any household disinfecting solution.
Important: Contact your healthcare provider or local hemophilia treatment center if you experience any problems.
BAXALTA®, ADVATE®, BAXJECT® and RECOMBINATE® are trademarks of Baxalta Incorporated, a wholly-owned, indirect subsidiary of Shire plc.
SHIRE and the Shire Logo are trademarks or registered trademarks of Shire Pharmaceutical Holdings Ireland Limited or its affiliates.
Patented: see https://www.shire.com/legal-notice/product-patents
Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020Issued 12/2018
-
PRINCIPAL DISPLAY PANEL - 250 IU 2 mL Kit Carton
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3051-02
Single use only
For Intravenous Administration Only
Rx OnlyLyophilized powder for reconstitution
ADVATE and diluent preassembled in BAXJECT III system
Product does not contain plasma/albumin
ACTUAL POTENCY
2
mLShire
-
PRINCIPAL DISPLAY PANEL - 250 IU 2 mL Blister Pack Label
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3051-03
Single use only
For Intravenous Administration Only
ADVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
2
mLProduct does not contain plasma/albumin
Shire
0742472
IU:
LOT:
EXP:
- PRINCIPAL DISPLAY PANEL - 250 IU 2 mL Container Label
-
PRINCIPAL DISPLAY PANEL - 500 IU 2 mL Kit Carton
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3052-02
Single use only
For Intravenous Administration Only
Rx OnlyLyophilized powder for reconstitution
ADVATE and diluent preassembled in BAXJECT III system
Product does not contain plasma/albumin
ACTUAL POTENCY
2
mLShire
-
PRINCIPAL DISPLAY PANEL - 500 IU 2 mL Blister Pack Label
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3052-03
Single use only
For Intravenous Administration Only
ADVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
2
mLProduct does not contain plasma/albumin
Shire
0742476
IU:
LOT:
EXP:
- PRINCIPAL DISPLAY PANEL - 500 IU 2 mL Container Label
-
PRINCIPAL DISPLAY PANEL - 1000 IU 2 mL Kit Carton
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3053-02
Single use only
For Intravenous Administration Only
Rx OnlyLyophilized powder for reconstitution
ADVATE and diluent preassembled in BAXJECT III system
Product does not contain plasma/albumin
ACTUAL POTENCY
2
mLShire
-
PRINCIPAL DISPLAY PANEL - 1000 IU 2 mL Blister Pack Label
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3053-03
Single use only
For Intravenous Administration Only
ADVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
2
mLProduct does not contain plasma/albumin
Shire
0742479
IU:
LOT:
EXP:
- PRINCIPAL DISPLAY PANEL - 1000 IU 2 mL Container Label
-
PRINCIPAL DISPLAY PANEL - 1500 IU 2 mL Kit Carton
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3054-02
Single use only
For Intravenous Administration Only
Rx OnlyLyophilized powder for reconstitution
ADVATE and diluent preassembled in BAXJECT III system
Product does not contain plasma/albumin
ACTUAL POTENCY
2
mLShire
-
PRINCIPAL DISPLAY PANEL - 1500 IU 2 mL Blister Pack Label
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3054-03
Single use only
For Intravenous Administration Only
ADVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
2
mLProduct does not contain plasma/albumin
Shire
0742482
IU:
LOT:
EXP:
- PRINCIPAL DISPLAY PANEL - 1500 IU 2 mL Container Label
-
PRINCIPAL DISPLAY PANEL - 2000 IU 5 mL Kit Carton
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3045-10
Single use only
For Intravenous Administration Only
Rx OnlyLyophilized powder for reconstitution
ADVATE and diluent preassembled in BAXJECT III system
Product does not contain plasma/albumin
ACTUAL POTENCY
5
mLShire

-
PRINCIPAL DISPLAY PANEL - 2000 IU 5 mL Blister Pack Label
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3045-11
Single use only
For Intravenous Administration Only
ADVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
5
mLProduct does not contain plasma/albumin
Shire
0742485
IU:
LOT:
EXP:
- PRINCIPAL DISPLAY PANEL - 2000 IU 5 mL Container Label
-
PRINCIPAL DISPLAY PANEL - 3000 IU 5 mL Kit Carton
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3046-10
Single use only
For Intravenous Administration Only
Rx OnlyLyophilized powder for reconstitution
ADVATE and diluent preassembled in BAXJECT III system
Product does not contain plasma/albumin
ACTUAL POTENCY
5
mLShire
-
PRINCIPAL DISPLAY PANEL - 3000 IU 5 mL Blister Pack Label
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3046-11
Single use only
For Intravenous Administration Only
ADVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
5
mLProduct does not contain plasma/albumin
Shire
0742488
IU:
LOT:
EXP:
- PRINCIPAL DISPLAY PANEL - 3000 IU 5 mL Container Label
-
PRINCIPAL DISPLAY PANEL - 4000 IU 5 mL Kit Carton
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3047-10
Single use only
For Intravenous Administration Only
Rx OnlyLyophilized powder for reconstitution
ADVATE and diluent preassembled in BAXJECT III system
Product does not contain plasma/albumin
ACTUAL POTENCY
5
mLShire
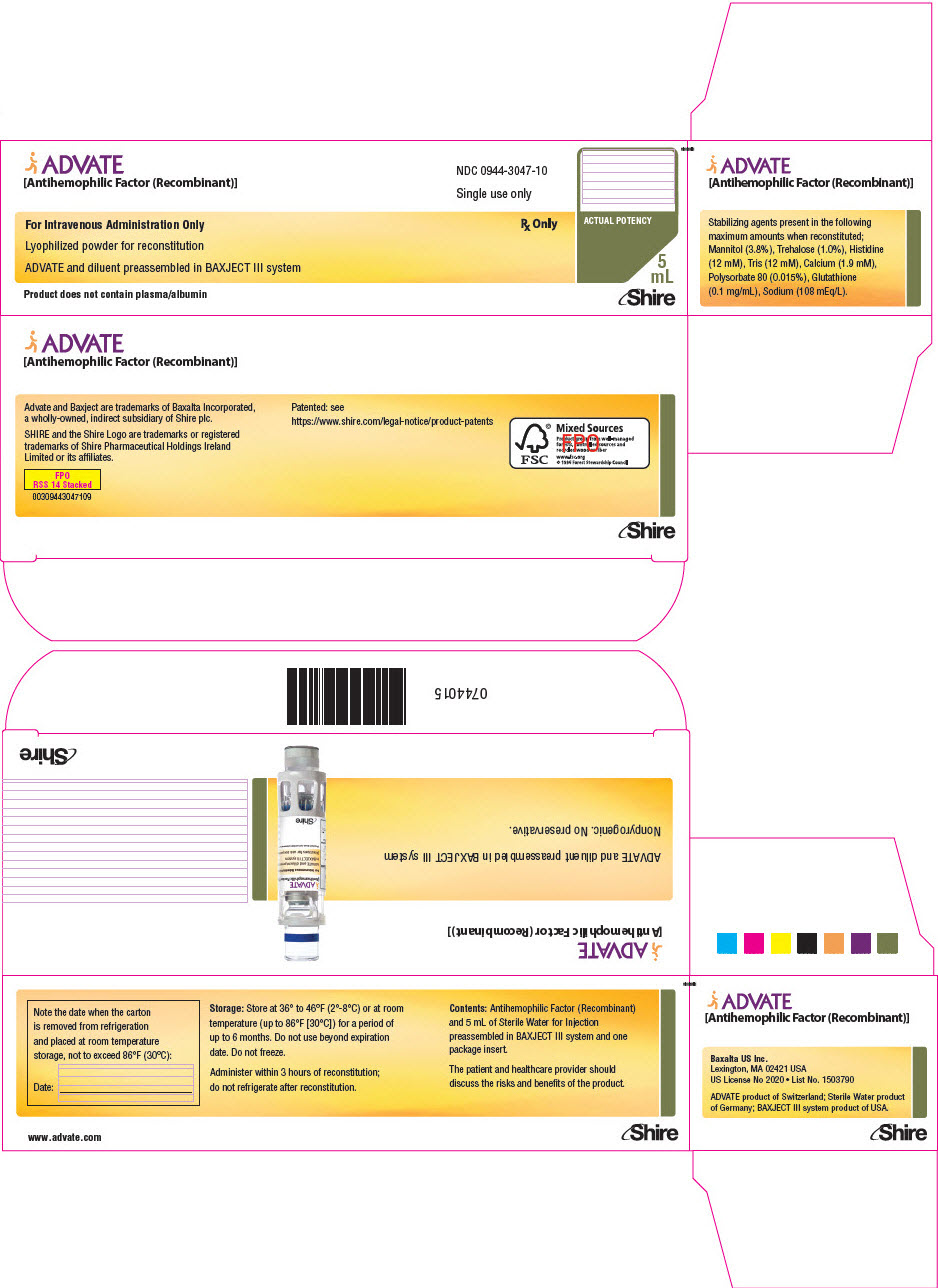
-
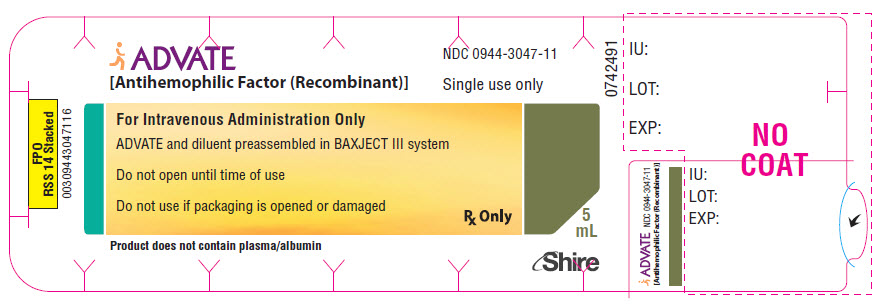
PRINCIPAL DISPLAY PANEL - 4000 IU 5 mL Blister Pack Label
ADVATE
[Antihemophilic Factor (Recombinant)]NDC: 0944-3047-11
Single use only
For Intravenous Administration Only
ADVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
5
mLProduct does not contain plasma/albumin
Shire
0742491
IU:
LOT:
EXP:
- PRINCIPAL DISPLAY PANEL - 4000 IU 5 mL Container Label
- PRINCIPAL DISPLAY PANEL - Advate Vial Label
- PRINCIPAL DISPLAY PANEL - Sterile Water for Injection Vial Label
-
INGREDIENTS AND APPEARANCE
ADVATE
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3051 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3051-02 1 in 1 CARTON 1 NDC: 0944-3051-03 1 in 1 BLISTER PACK 1 NDC: 0944-3051-04 1 in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 2 mL Part 1 of 2 ADVATE
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0944-2941 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 125 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTATHIONE (UNII: GAN16C9B8O) TREHALOSE (UNII: B8WCK70T7I) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2941-01 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 52919-008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2 mL in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52919-008-05 2 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 ADVATE
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3052 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3052-02 1 in 1 CARTON 1 NDC: 0944-3052-03 1 in 1 BLISTER PACK 1 NDC: 0944-3052-04 1 in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 2 mL Part 1 of 2 ADVATE
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0944-2942 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 250 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTATHIONE (UNII: GAN16C9B8O) TREHALOSE (UNII: B8WCK70T7I) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2942-02 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 52919-008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2 mL in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52919-008-05 2 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 ADVATE
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3053 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3053-02 1 in 1 CARTON 1 NDC: 0944-3053-04 1 in 1 BLISTER PACK 1 NDC: 0944-3053-03 1 in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 2 mL Part 1 of 2 ADVATE
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0944-2943 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTATHIONE (UNII: GAN16C9B8O) TREHALOSE (UNII: B8WCK70T7I) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2943-02 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 52919-008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 2 mL in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52919-008-05 2 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 ADVATE
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3054 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3054-02 1 in 1 CARTON 1 NDC: 0944-3054-03 1 in 1 BLISTER PACK 1 NDC: 0944-3054-04 1 in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 2 mL Part 1 of 2 ADVATE
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0944-2944 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 750 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTATHIONE (UNII: GAN16C9B8O) TREHALOSE (UNII: B8WCK70T7I) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2944-02 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Part 2 of 2 STERILE WATER
water injection, solutionProduct Information Item Code (Source) NDC: 52919-008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2 mL in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52919-008-05 2 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 12/15/2011 ADVATE
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3045 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3045-10 1 in 1 CONTAINER 1 NDC: 0944-3045-12 1 in 1 BLISTER PACK 1 NDC: 0944-3045-11 1 in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 ADVATE
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0944-2945 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 400 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTATHIONE (UNII: GAN16C9B8O) TREHALOSE (UNII: B8WCK70T7I) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2945-01 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 04/12/2006 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC: 0338-0001 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0001-56 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 04/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 04/12/2006 ADVATE
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3046 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3046-10 1 in 1 CARTON 1 NDC: 0944-3046-12 1 in 1 BLISTER PACK 1 NDC: 0944-3046-11 1 in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 ADVATE
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0944-2946 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 600 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTATHIONE (UNII: GAN16C9B8O) TREHALOSE (UNII: B8WCK70T7I) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2946-01 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 07/03/2007 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC: 0338-0001 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0001-56 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 07/03/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 07/03/2007 ADVATE
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3047 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3047-10 1 in 1 CARTON 1 NDC: 0944-3047-12 1 in 1 BLISTER PACK 1 NDC: 0944-3047-11 1 in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 ADVATE
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0944-2947 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 800 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTATHIONE (UNII: GAN16C9B8O) TREHALOSE (UNII: B8WCK70T7I) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-2947-01 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 07/12/2012 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC: 0338-0001 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0001-56 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 07/12/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125063 07/12/2012 Labeler - Baxalta US Inc. (079887619) Establishment Name Address ID/FEI Business Operations Baxalta US Inc. 009471603 MANUFACTURE Establishment Name Address ID/FEI Business Operations Baxalta Manufacturing Sarl 482124943 MANUFACTURE, API MANUFACTURE Establishment Name Address ID/FEI Business Operations Baxter AG 300434670 MANUFACTURE Establishment Name Address ID/FEI Business Operations Baxalta Manufacturing SARL Singapore Branch 595249573 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Siegfried Hameln GmbH 315869123 MANUFACTURE
Trademark Results [ADVATE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ADVATE 78075589 2867685 Live/Registered |
BAXALTA INCORPORATED 2001-07-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.