OCTAGAM IMMUNE GLOBULIN (HUMAN)- immune globulin solution
Octagam Immune Globulin (Human) by
Drug Labeling and Warnings
Octagam Immune Globulin (Human) by is a Prescription medication manufactured, distributed, or labeled by Octapharma Pharmazeutika Produktionsgesellschaft m.b.H.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
Octagam, Immune Globulin Intravenous (Human) 5% Liquid Preparation Initial U.S. Approval: 2004 - These highlights do not include all the information needed to use Octagam, Immune Globulin Intravenous (Human), safely and effectively. See full prescribing information for Octagam.
INDICATIONS AND USAGE
Octagam is an immune globulin intravenous (human), 5% liquid, indicated for treatment of primary humoral immunodeficiency (PI) (1). (1)
DOSAGE AND ADMINISTRATION
Intravenous use only (2). (2)
(2)
Indication Dose Initial Infusion rate Maintenance infusion rate (if tolerated) PI (2)
300-600mg/kg (2)
0.5mg/kg/min (2)
3.33 mg/kg/min (2)
Every 3-4 weeks (2)
Ensure that patients with pre-existing renal insufficiency are not volume depleted; discontinue Octagam 5% liquid if renal function deteriorates (2.4). (2)
For patients at risk of renal dysfunction or thrombotic events, administer Octagam 5% liquid at the minimum infusion rate practicable (2.4). (2)
DOSAGE FORMS AND STRENGTHS
Octagam 5% liquid is supplied in 1.0 g, 2.5 g, 5 g , 10 g or 25 g single use bottles (3, 16) (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
IgA deficient patients with antibodies against IgA are at greater risk of developing severe hypersensitivity and anaphylactic reactions Epinephrine should be available immediately to treat any acute severe hypersensitivity reactions. (5.1) (5)
Monitor renal function, including blood urea nitrogen and serum creatinine, and urine output in patients at risk of developing acute renal failure. (5.2) (5)
Falsely elevated blood glucose readings may occur during and after the infusion of Octagam 5% liquid with some glucometer and test strip systems (5.3) (5)
Hyperproteinemia, increased serum viscosity and hyponatremia occur in patients receiving IGIV therapy. (5.4) (5)
Thrombotic events have occurred in patients receiving IGIV therapy. Monitor patients with known risk factors for thrombotic events; consider baseline assessment of blood viscosity for those at risk of hyperviscosity. (5.5) (5)
Aseptic Meningitis Syndrome has been reported with Octagam 5% liquid and other IGIV treatments, especially with high doses or rapid infusion. (5.6) (5)
Hemolytic anemia can develop subsequent to IGIV therapy due to enhanced RBC sequestration (5.7) (5)
IGIV recipients should be monitored for pulmonary adverse reactions (TRALI) (5.8) (5)
The product is made from human plasma and may contain infectious agents, e.g. viruses and, theoretically, the Creutzfeldt-Jakob disease agent (5.9) (5)
ADVERSE REACTIONS
Most common adverse reactions with an incidence of > 5% during a clinical trial were headache and nausea. (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Octapharma at 1-866-766-4860 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pregnancy: no human or animal data. Use only if clearly needed (8.1). (8)
In patients over age 65 or in any person at risk of developing renal insufficiency, do not exceed the recommended dose, and infuse Octagam 5% liquid at the minimum infusion rate practicable (8.5). (8)
(8)
See 17 for PATIENT COUNSELING INFORMATION. (8)
(8)
Revised: September 2009 (8)
(8)
(8)
(8)
_______________________________________________________________________________________________________________________________________ (8)
(8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2009
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
INDICATIONS AND USAGE
Primary Humoral Immunodeficiency Diseases (PI)
DOSAGE AND ADMINISTRATION
Preparation and handling
Treatment of Primary Humoral Immunodeficiency
Missed Doses
Administration
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Sensitivity
Renal Failure
Blood Glucose Monitoring
Hyperproteinemia
Thrombotic events
Aseptic meningitis syndrome
Hemolysis
Transfusion-Related Acute Lung Injury (TRALI)
General
Laboratory Tests
ADVERSE REACTIONS
Adverse Drug Reaction Overview
Clinical Trials Experience
Postmarketing Experience
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pregnancy
Nursing Mothers
Pediatric Use
Geriatric Use
OVERDOSAGE
DESCRIPTION
Composition
CLINICAL PHARMACOLOGY
Mechanism of Action
Pharmacodynamics
Pharmacokinetics
NON-CLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal Toxicology and/or Pharmacology
CLINICAL STUDIES
REFERENCES
PATIENT COUNSELING INFORMATION
Information for Patients
- * Sections or subsections omitted from the full prescribing information are not listed.
-
INDICATIONS AND USAGE
Primary Humoral Immunodeficiency Diseases (PI)
Octagam is an immune globulin intravenous (human) 5% liquid indicated for treatment of primary humoral immunodeficiency (PI), such as congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome and severe combined immunodeficiencies.
-
DOSAGE AND ADMINISTRATION
For intravenously use only
Preparation and handling
- Octagam 5% liquid should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if turbid and/or discoloration is observed.
- Octagam 5% liquid must not be mixed with other medicinal products or administered simultaneously with other intravenous preparation in the same infusion set. Do not mix with immune globulin intravenous (IGIV) products from other manufacturers.
- Do not freeze. Solutions that have been frozen should not be used.
- Octagam 5% liquid bottle is for single use only. Octagam 5% liquid contains no preservative. Any bottle that has been entered should be used promptly. Partially used bottles should be discarded.
- Content of Octagam 5% liquid bottles may be pooled under aseptic conditions into sterile infusion bags and infused within 8 hours after pooling.
- Do not use after expiration date.
- Octagam 5% liquid should not be diluted.
Treatment of Primary Humoral Immunodeficiency
As there are significant differences in the half-life of IgG among patients with primary humoral immunodeficiencies, the frequency and amount of immunoglobulin therapy may vary from patient to patient. The proper amount can be determined by monitoring clinical response.
The dose of Octagam 5% liquid for replacement therapy in primary humoral immunodeficiency diseases is 300 to 600 mg/kg body weight (6-12ml/kg) administered every 3 to 4 weeks. The dosage may be adjusted over time to achieve the desired trough levels and clinical responses.
If a patient is at risk of measles exposure (ie., outbreak in US or travel to endemic areas outside of the US) and receives a dose of less than 400 mg/kg every 3 to 4 weeks, the dose should be increased to at least 400 mg/kg. If a patient has been exposed to measles, this dose should be administered as soon as possible after exposure.
Missed Doses
If a patient on regular treatment missed a dose, the missed dose should be administered as soon as possible, and then treatment should continue as before.
Administration
Octagam 5% liquid should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if turbid and/or discoloration is observed.
Octagam 5% liquid should be at room temperature during administration. Only administer intravenously.
Any bottle that has been opened should be used promptly. Partially used bottles should be discarded.
Octagam 5% liquid is not supplied with an infusion set. If an in-line filter is used the pore size should be 0.2 – 200 microns.
Do not use a needle of larger than 16 gauge to prevent the possibility of coring. Insert needle only once, within the stopper area delineated (by the raised ring for penetration). The stopper should be penetrated perpendicular to the plane of the stopper within the ring.
Rate of Administration
It is recommended that Octagam 5% liquid be initially infused at infusion rates stated below, at least until the physician has had adequate experience with a given patient.
Infusion rates: 0.5mg/kg/min (30mg/kg/hr for the first 30 minutes; if tolerated, advance to 1mg/kg/min (60mg/kg/hr) for the second 30 minutes; and if further tolerated, advance to 2mg/kg/min (120mg/kg/hr) for the third 30 minutes. Thereafter the infusion can be maintained at a rate up to, but not exceeding, 3.33mg/kg/min (200mg/kg/hr)
For patients judged to be at risk for developing renal dysfunction, administer Octagam 5% liquid at the minimum infusion rate practicable, not to exceed 0.07 ml/kg (3.3 mg/kg)/minute (200 mg/kg/hour).
Table 1
Rate of Administration mg/kg/min (mg/kg/hour) ml/kg/min first 30 min
0.5 (30)
0.01
next 30 min
1.0 (60)
0.02
next 30 min
2.0 (120)
0.04
Maximum
< 3.33 (<200)
<0.07
Certain severe adverse drug reactions may be related to the rate of infusion. Slowing or stopping the infusion usually allows the symptoms to disappear promptly.
Ensure that patients with pre-existing renal insufficiency are not volume depleted; discontinue Octagam 5% liquid if renal function deteriorates.
For patients at risk of renal dysfunction or thromboembolic events, administer Octagam 5% liquid at the minimum infusion rate practicable.
Incompatibilities
Octagam 5% liquid must not be mixed with other medicinal products or administered simultaneously with other intravenous preparations in the same infusion set.
Shelf-life
Octagam 5% liquid may be stored for 24 months at +2°C to + 25°C (36°F to 77°F) from the date of manufacture.
Special Precautions for Storage
Do not freeze. Frozen product should not be used.
Do not use after expiration date.
- DOSAGE FORMS AND STRENGTHS
-
CONTRAINDICATIONS
Octagam 5% liquid is contraindicated in patients who have acute severe hypersensitivity reactions to human immunoglobulin.
Octagam 5% liquid contains trace amounts of IgA (not more than 0.2 mg/ml in a 5% solution). It is contraindicated in IgA deficient patients with antibodies against IgA and history of hypersensitivity (See Description [11]).
Octagam 5% liquid is contraindicated in patients with acute hypersensitivity reaction to corn. Octagam 5% liquid contains maltose, a disaccharide sugar which is derived from corn. Patients known to have corn allergies should avoid using Octagam 5% liquid.
-
WARNINGS AND PRECAUTIONS
Sensitivity
Severe hypersensitivity reactions may occur [ 1 ] (See Contraindications [4.1]). In case of hypersensitivity, Octagam 5% liquid infusion should be immediately discontinued and appropriate treatment instituted. Epinephrine should be immediately available for treatment of acute severe hypersensitivity reaction. IgA deficient patients with antibodies against IgA are at greater risk of developing severe hypersensitivity and anaphylactoid reactions when administered Octagam 5% liquid (See Contraindications [4.2]). Patients known to have corn allergies should avoid using Octagam 5% liquid (See Contraindications [4.3]).
Renal Failure
Assure that patients are not volume depleted prior to the initiation of the infusion of Octagam 5% liquid.
Periodic monitoring of renal function tests and urine output is particularly important in patients judged to have a potential increased risk of developing acute renal failure. Renal function, including a measurement of blood urea nitrogen (BUN)/serum creatinine, should be assessed prior to the initial infusion of Octagam 5% liquid and again at appropriate intervals thereafter. If renal function deteriorates, discontinuation of the product should be considered (See Patient Counselling Information [17])
For patients judged to be at risk for developing renal dysfunction and/or at risk of developing thrombotic events, it may be prudent to reduce the amount of product infused per unit time by infusing Octagam 5% liquid at a maximum rate less than 0.07 ml/kg (3.3 mg/kg)/minute (200 mg/kg/hour) (See Boxed Warning, and Dosage and Administration [2.4]).
Blood Glucose Monitoring
Blood Glucose Testing [ 2 ] :some types of blood glucose testing systems (for example, those based on the glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase methods) falsely interpret the maltose contained in Octagam 5% liquid as glucose. This has resulted in falsely elevated glucose readings and, consequently, in the inappropriate administration of insulin, resulting in life-threatening hypoglycemia. Also, cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated glucose readings. Accordingly, when administering Octagam 5% liquid, the measurement of blood glucose must be done with a glucose-specific method. The product information of the blood glucose testing system, including that of the test strips, should be carefully reviewed to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, contact the manufacturer of the testing system to determine if the system is appropriate for use with maltose-containing parenteral products.
Hyperproteinemia
Hyperproteinemia, increased serum viscosity and hyponatremia may occur in patients receiving IGIV therapy. The hyponatremia is likely to be a pseudohyponatremia as demonstrated by a decreased calculated serum osmolality or elevated osmolar gap. Distinguishing true hyponatremia from pseudohyponatremia is clinically critical, as treatment aimed at decreasing serum free water in patients with pseudohyponatremia may lead to volume depletion, a further increase in serum viscosity and a disposition to thromboembolic events [ 3 ].
Thrombotic events
Thrombotic events have been reported in association with IGIV therapy [ 4 ],[ 5 ],[ 6 ]. Patients at risk may include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization and/or known or suspected hyperviscosity. The potential risks and benefits of IGIV should be weighed against those of alternative therapies for all patients for whom IGIV administration is being considered. Baseline assessment of blood viscosity should be considered in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia / markedly high triacylglycerols (triglycerides), or monoclonal gammopathies.
Aseptic meningitis syndrome
Aseptic meningitis syndrome (AMS) has been reported to occur infrequently in association with IGIV treatment. Discontinuation of IGIV treatment has resulted in remission of AMS within several days without sequelae. The syndrome usually begins within several hours to two days following IGIV treatment and rapid infusion. It is characterized by symptoms and signs including severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea and vomiting. Cerebrospinal fluid (CSF) studies are frequently positive with pleocytosis up to several thousand cells per cu mm, predominantly from the granulocytic series, and elevated protein levels up to several hundred mg/dl. Patients exhibiting such symptoms and signs should receive a thorough neurological examination, including CSF studies, to rule out other causes of meningitis. It appears that patients with a history of migraine may be more susceptible. [ 7 ] (See Patient Counselling Information [17]).
Hemolysis
IGIV products can contain blood group antibodies which may act as hemolysins and induce in vivo coating of red blood cells with immunoglobulin, causing a positive direct antiglobulin reaction and, rarely, hemolysis [ 8 ]. Hemolytic anemia can develop subsequent to IGIV therapy due to enhanced RBC sequestration [See ADVERSE REACTIONS] [ 9 ]. IGIV recipients should be monitored for clinical signs and symptoms of hemolysis. If signs and/or symptoms of hemolysis are present after IGIV infusion, appropriate confirmatory laboratory testing should be done (See Patient Counseling Information [17]).
Transfusion-Related Acute Lung Injury (TRALI)
There have been reports of noncardiogenic pulmonary edema [Transfusion-Related Acute Lung Injury (TRALI)] in patients administered IGIV [ 10 ]. TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever and typically occurs within 1-6 hours after transfusion. Patients with TRALI may be managed using oxygen therapy with adequate ventilatory support.
IGIV recipients should be monitored for pulmonary adverse reactions (See Patient Counseling Information [17]). If TRALI is suspected, appropriate tests should be performed for the presence of anti-neutrophil antibodies in both the product and patient serum.
General
Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Octapharma. The physician should discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient (See Patient Counseling Information [17]).
Laboratory Tests
If signs and/or symptoms of hemolysis are present after IGIV infusion, appropriate confirmatory laboratory testing should be done.
If TRALI is susppected, appropriate tests should be performed for the presence of anti-neutrophil antibodies in both the product and patient serum.
Because of the potentially increased risk of thrombosis, baseline assessment of blood viscosity should be considered in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies.
-
ADVERSE REACTIONS
Adverse Drug Reaction Overview
The most serious adverse reactions observed with Octagam 5% Liquid treatment have been immediate anaphylactic reactions, aseptic meningitis, and hemolytic anemia.
The most common adverse reactions observed with Octagam 5% Liquid treatment during clinical trial (> 5%) were headache and nausea.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in clinical practice.
The clinical trial database includes a multi-center, open-label, single arm study in 46 children and adults with PI. Subjects participated in the study for a mean of 346 days and received 300 to 450 mg/kg every 21 days or 400 to 600 mg/kg every 28 days. Infusions were initiated at a rate of 30 mg/kg/hour for the first 30 minutes, and, if tolerated, could be advanced to a maximum tolerated rate not exceeding 200 mg/kg/hour. Over half of the subjects were male (n=28; 61%), and more than half were on the 28-day infusion schedule (n=27; 59%). The mean age of subjects was 31.5 years.
Six subjects experienced a total of 12 SAEs (abdominal pain (2 occurrences), cardiac arrest, pneumonia, cellulitis, coxsackie viral infection, renal calculus (2 occurrences), blood culture positive, ketonuria, gastroenteritis, and colitis pseudomembranosus). Eleven of the 12 SAEs were not suspected to be related to study drug; the other SAE was noted before the subject began receiving the next scheduled infusion, and it was not temporally related to the previous infusion.
Pre-medications were used in 165 (25.2%) out of 654 infusions and in 14 (30.4%) out of 46 patients. Infusions were slowed or interrupted in 9 out of 489 infusions (1.84%) without pre-medication and in 10 out of 165 infusions (6.06%) with pre-medication. Five out of 32 (15.63%) patients who never received any pre-medication had at least one slowed or interrupted infusion, whereas 9 out of 14 (64.29%) patients who received pre-medication at least once also had a slowed or interrupted infusion.
Six of the 46 subjects in the trial (13%) were withdrawn from the study: 2 on the subjects' request; 1 because of investigator's decision (non-compliance); 1 because of loss to follow-up; 1 death (cardiac arrest, not suspected to be related to study drug); and 1 by error of the study coordinator.
All adverse events in trial OCTA-06, irrespective of the causality assessment, reported by at least 5% of subjects during the 12-months treatment are given in the table below.
Table 2: Subjects and Infusions with at least one Adverse Event Irrespective of Causality (Study OCTA-06)
Octagam 5% liquid No. of subjects (%)
No. of infusions (%)
Total
46 (100%)
654 (100%)
Nasal congestion
24 (52%)
39 (6%)
Sinusitis NOS
23 (50%)
45 (7%)
Headache NOS
22 (48%)
62 (9%)
Cough
20 (43%)
46 (7%)
Sore throat NOS
16 (35%)
25 (4%)
Fever
15 (33%)
19 (3%)
Vomiting NOS
12 (26%)
15 (2%)
Diarrhoea NOS
11 (24%)
22 (3%)
Bronchitis NOS
10 (22%)
14 (2%)
Abdominal pain upper
9 (20%)
11 (2%)
Arthralgia
9 (20%)
15 (2%)
Nasopharyngitis
8 (17%)
14 (2%)
Rhinorrhoea
8 (17%)
9 (1%)
Upper respiratory tract infection NOS
8 (17%)
13 (2%)
Fatigue
7 (15%)
9 (1%)
Nausea
7 (15%)
8 (1%)
Pain in limb
7 (15%)
10 (2%)
Sinus congestion
7 (15%)
9 (1%)
Back pain
5 (11%)
10 (2%)
Injection site reaction NOS
5 (11%)
11 (2%)
Wheezing
5 (11%)
8 (1%)
Asthma NOS
4 (9%)
5 (0.8%)
Asthma aggravated
4 (9%)
10 (2%)
Chest pain NEC
4 (9%)
4 (0.6%)
Conjunctivitis NEC
4 (9%)
4 (0.6%)
Dyspepsia
4 (9%)
5 (0.8%)
Earache
4 (9%)
6 (0.9%)
Ecchymosis
4 (9%)
7 (1%)
Fungal infection NOS
4 (9%)
4 (0.6%)
Injection site pain
4 (9%)
4 (0.6%)
Insomnia NEC
4 (9%)
4 (0.6%)
Sinusitis acute NOS
4 (9%)
8 (1%)
Urinary tract infection NOS
4 (9%)
8 (1%)
Vaginal candidiasis
4 (9%)
7 (1%)
Abdominal pain NOS
3 (7%)
3 (0.5%)
Dizziness (exc vertigo)
3 (7%)
4 (0.6%)
Dyspnoea NOS
3 (7%)
3 (0.5%)
Epistaxis
3 (7%)
5 (0.8%)
Eye discharge
3 (7%)
3 (0.5%)
Eye irritation
3 (7%)
3 (0.5%)
Hypertension NOS
3 (7%)
5 (0.8%)
Otitis media NOS
3 (7%)
4 (0.6%)
Pain NOS
3 (7%)
4 (0.6%)
Postnasal drip
3 (7%)
3 (0.5%)
Productive cough
3 (7%)
3 (0.5%)
Rigors
3 (7%)
4 (0.6%)
Throat irritation
3 (7%)
3 (0.5%)
Urticaria NOS
3 (7%)
8 (1%)
The adverse reactions in trial OCTA-06 reported by at least 5% of subjects during the 12-month treatment are given in the table below.
Table 3: Subjects and Infusions with At Least One Adverse Reaction (Study OCTA-06)
Octagam 5% liquid No. of subjects (%)
No. of infusions (%)
Total
46 (100%)
654 (100%)
Headache NOS
7 (15%)
18 (3%)
Nausea
3 (7%)
4 (0.6%)
The following table provides an overview on the temporally associated adverse events (TAAEs) during and within different time-points after the end of Octagam infusion.
Table 4: Overview on TAAEs Occurring During and Over a Specified Number of Hours after the End of Infusion, Irrespective of Causality (Study OCTA-06)
Total # of infusions (N=654) Time-Points 24 hrs
48hrs
72hrs
Total # of TAAEs
172
183
189
Proportion of infusions with TAAEs
26.3%
28.0%
28.9%
Upper bound 1 sided 97.5% CI for proportion of TAAEs
29.7%
31.4%
32.4%
All temporally associated adverse events (TAAEs) in trial OCTA-06, irrespective of the causality assessment, reported by at least 5% of subjects within 72 hours after the end of the infusion are given in the table below.
Table 5: TAAEs During and Over 72 Hours After End of Infusion, Irrespective of Causality (Study OCTA-06)
TAAE Subjects (%)n=46 Infusion (%)N=654 Headache NOS
15 (32.6%)
28 (4.3%)
Sinusitis NOS
12 (26.1%)
13 (2.0%)
Nasal congestion
10 (21.7%)
11 (1.7%)
Arthralgia
7 (15.2%)
10 (1.5%)
Cough
7 (15.2%)
7 (1.1%)
Injection site reaction NOS
5 (10.9%)
11 (1.7%)
Sore throat NOS
5 (10.9%)
5 (0.8%)
Vomiting NOS
5 (10.9%)
5 (0.8%)
Back pain
4 (8.7%)
6 (0.9%)
Diarrhoea NOS
4 (8.7%)
5 (0.8%)
Ecchymosis
4 (8.7%)
5 (0.8%)
Injection site pain
4 (8.7%)
4 (0.6%)
Nausea
4 (8.7%)
5 (0.8%)
Upper respiratory tract infection NOS
4 (8.7%)
5 (0.8%)
Wheezing
4 (8.7%)
6 (0.9%)
Asthma aggravated
3 (6.5%)
4 (0.6%)
Eye irritation
3 (6.5%)
3 (0.5%)
Fungal infection NOS
3 (6.5%)
3 (0.5%)
Pain in limb
3 (6.5%)
5 (0.8%)
Rhinorrhoea
3 (6.5%)
3 (0.5%)
Urinary tract infection NOS
3 (6.5%)
3 (0.5%)
The subset of drug related temporally associated adverse events (TAAEs) in trial OCTA-06 reported by at least 5% of subjects within 72 hours after the end of the infusion is given in the table below.
Table 6: Drug-Related TAAEs During and Over 72 Hours After End of Infusion (Study OCTA-06)
TAAE Subjects (%)n=46 Infusion (%)N=654 Headache NOS
6 (13.0%)
15 (2.3%)
Nausea
3 (6.5%)
4 (0.6%)
Laboratory Abnormalities
Standard clinical laboratory evaluations were performed Study OCTA-06. Three subjects (7%) had incidences of AST (>2.5 x ULN) which were all assessed as clinically non-significant. Four subjects (9%) had incidences of serum creatinine increases being stable throughout the course of the study. Therefore, these observations were not regarded as indicative of acute renal dysfunction.
Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
Octagam 5% liquid Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Octagam 5% liquid. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to Octagam 5% liquid.
Blood and lymphatic system disordersLeukopenia, haemolytic anaemia Immune system disorders
Hypersensitivity, anaphylactic shock, anaphylactic reaction, anaphylactoid reaction, angioneurotic oedema, face oedema
Metabolic and nutritional disorders
Fluid overload
Psychiatric disorders
Agitation
Nervous system disorders
Headache, cerebrovascular accident, meningitis aseptic, migraine, dizziness, paraesthesia
Cardiac disorders
Myocardial infarction, tachycardia, palpitations, cyanosis
Vascular disorders
Hypotension, thrombosis, peripheral circulatory failure, hypertension
Respiratory, thoracic and mediastinal disorders
Respiratory failure, pulmonary embolism, pulmonary oedema, bronchospasm, dyspnoea, cough
Gastrointestinal disorders
Nausea, vomiting, diarrhoea, abdominal pain
Skin and subcutaneous tissue disorders
Eczema, urticaria, rash, rash erythematous, dermatitis, pruritus, alopecia
Musculoskeletal and connective tissue disorders
Back pain, arthralgia, myalgia, pain in extremity
Renal and urinary disorders
Renal failure acute
General disorders and administration site conditions
Fatigue, injection site reaction, pyrexia, chills, chest pain, hot flush, flushing, hyperhidrosis, malaise
Investigations
Hepatic enzymes increased, blood glucose false positive
General
The following adverse reactions have been identified during post-approval use of IGIV products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to IGIV products:
RespiratoryApnea, Acute Respiratory Distress Syndrome (ARDS), Transfusion Related Acute Lung Injury (TRALI), cyanosis, hypoxemia, pulmonary edema, dyspnea, bronchospasm Cardiovascular
Cardiac arrest, thromboembolism, vascular collapse, hypotension
Neurological
Coma, loss of consciousness, seizures, tremor
Integumentary
Steven-Johnson syndrome, epidermolysis, erythema multiforme, bullous dermatitis
Hematologic
Pancytopenia, leukopenia, hemolysis, positive direct antiglobulin (Coombs) test
General / Body as a Whole
Pyrexia, rigors
Musculoskeletal
Back pain
Gastrointestinal
Hepatic dysfunction, abdominal pain
-
DRUG INTERACTIONS
Admixtures of Octagam 5% liquid with other drugs and intravenous solutions have not been evaluated. It is recommended that Octagam 5% liquid be administered separately from other drugs or medications which the patient may be receiving. The product should not be mixed with IGIVs from other manufacturers.
The infusion line may be flushed before and after administration of Octagam 5% liquid with either normal saline or 5% dextrose in water.
Various passively transferred antibodies in immunoglobulin preparations can confound the results of serological testing.
Antibodies in Octagam 5% liquid may interfere with the response to live viral vaccines, such as measles, mumps, and rubella. Physicians should be informed of recent therapy with IGIVs, so that administration of live viral vaccines, if indicated, can be appropriately delayed 3 or more months from the time of IGIV administration.
-
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with Octagam 5% liquid. It is also not known whether Octagam 5% liquid can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Octagam 5% liquid should be given to a pregnant woman only if clearly needed.
Pediatric Use
Octagam 5% liquid was evaluated in 11 pediatric subjects (age range 6 – 16 years). There were no obvious differences observed between adults and pediatric subjects with respect to pharmacokinetics, efficacy and safety. No pediatric specific dose requirements were necessary to achieve the desired serum IgG levels.
Geriatric Use
Patients > 65 years of age may be at increased risk for developing certain adverse reactions such as thromboembolic events and acute renal failure (See Boxed Warnings and Precautions [5.3]). In the clinical trial only 4 geriatric patients ( > 65 years) were enrolled, a number insufficient to determine whether geriatric patients respond differently from younger subjects. In these 4 patients no particular issues were observed.
- OVERDOSAGE
-
DESCRIPTION
Immune Globulin Intravenous (Human), Octagam 5% liquid, is a solvent/detergent (S/D)-treated, sterile preparation of highly purified immunoglobulin G (IgG) derived from large pools of human plasma. Octagam 5% liquid is a solution for infusion which must be administered intravenously.
All units of human plasma used in the manufacture of Octagam 5% liquid are provided by FDA-approved blood establishments only, and are tested by FDA-licensed serological tests for HBsAg, antibodies to HCV and HIV and Nucleic Acid Test (NAT) for HCV and HIV-1 and found to be non-reactive (negative).
The product is manufactured by the cold ethanol fractionation process followed by ultrafiltration and chromatography. The manufacturing process includes treatment with an organic S/D mixture composed of tri-n-butyl phosphate (TNBP) and Triton X-100 (Octoxynol). The Octagam 5% liquid manufacturing process provides a significant viral reduction in in vitro studies (table 7). These reductions are achieved through a combination of process steps including cold ethanol fractionation, S/D treatment and pH 4 treatment.
Table 7: In vitro reduction factor during Octagam 5% liquid manufacturing
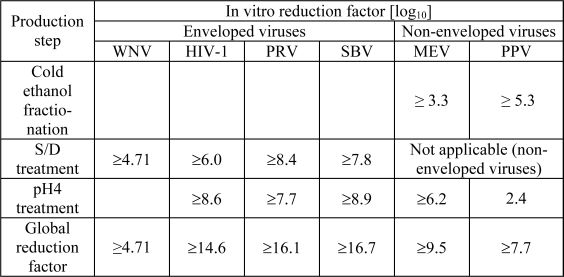
WNV: West Nile Virus
HIV-1: Human Immunodeficiency Virus - 1
PRV: Pseudorabies Virus
SBV: Sindbis Virus
MEV: Mouse Encephalomyelitis Virus
PPV: Porcine Parvovirus
Composition
The composition of Octagam 5% liquid is shown in table 8 as follows:
Table 8 : Composition
Component Quantity/ml Protein, of which not less than 96% is human normal immunoglobulin G
50 mg
Maltose
100 mg
Triton X-100
not more than 5 mcg
TNBP
not more than 1 mcg
IgA
not more than 0.2 mg
IgM
not more than 0.1 mg
Water for Injection
ad.
This preparation contains approximately 50 mg of protein per ml (5%) of which not less than 96% is human normal immunoglobulin G. Octagam 5% liquid contains not more than 3% aggregates, not less than 90% monomers and dimers and not more than 3% fragments.
The sodium content of the final solution is not more than 30 mmol/l and the pH is between 5.1 and 6.0. The osmolality is 310 - 380 mosmol/kg.
The manufacturing process for Octagam 5% liquid isolates IgG without additional chemical or enzymatic modification, and the Fc portion is maintained intact Octagam 5% liquid contains the IgG antibody activities present in the donor population. IgG subclasses are fully represented with the following approximate percents of total IgG: IgG 1 is 65%, IgG 2 is 30%, IgG 3 is 3% and IgG 4 is 2%.
Octagam 5% liquid contains a broad spectrum of IgG antibodies against bacterial and viral agents that are capable of opsonization and neutralization of microbes and toxins.
Octagam 5% liquid contains no preservative and no sucrose.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Octagam 5% liquid supplies a broad spectrum of opsonic and neutralizing IgG antibodies against bacteria or their toxins. The mechanism of action in PI has not been fully elucidated.
Pharmacodynamics
Octagam 5% liquid contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against various infectious agents reflecting the IgG activity found in the donor population. Octagam 5% liquid which is prepared from pooled material from not less than 1000 donors, has an IgG subclass distribution similar to that of native human plasma. Adequate doses of IGIV can restore abnormally low IgG level to the normal range. Standard pharmacodynamic studies were not performed.
Pharmacokinetics
Peak levels of IgG are reached immediately after infusion of Octagam 5% liquid. It has been shown that after infusion, exogenous IgG is distributed relatively rapidly between plasma and extravascular fluid until approximately half is partitioned in the extravascular space. Therefore a rapid initial drop in serum IgG is expected [ 11 ].
Studies show that the apparent half-life of Octagam 5% liquid is approximately 40 days in immunodeficient patients.
The main pharmacokinetic parameters of Octagam 5% liquid measured as total IgG in study OCTA-06 are displayed below:
In the pharmacokinetic study, a subset of 14 patients aged between 10 and 70 years with PI underwent pharmacokinetic assessments. Patients received infusions of Octagam 5% liquid (300 to 600 mg/kg) every 3 (n=6) to 4 (n=8) weeks for 12 months. Pharmacokinetic samples were collected at baseline and after the 5 th month of treatment. After the infusion, blood samples were taken until day 28 (for patients on a 21 day schedule, the interval was extended to 4 weeks for the pharmacokinetic study).
Table 9: PK Parameters of Octagam 5% liquid (Study OCTA-06)
Octagam 5% liquid N
Mean
SD
Median
Cmax (mg/mL)
14
16.7
3.2
16.4
AUC (mg*h/mL)
14
7022
1179
7103
T1/2 (days)
14
40.7
17.0
36.3
Trough IgG Level
21 Day Infusion Schedule (mg/dL)
19
881.6
151.5
859
Trough IgG Level
28 Day Infusion Schedule (mg/dL)
25
763.5
156.8
760
The half-life of IgG can vary considerably from person to person. In particular, high concentrations of lgG and hypermetabolism associated with fever and infection have been seen to coincide with a shortened half-life of IgG. Longer half-lives are often seen with immunodeficient patients [ 12 ].
-
NON-CLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies were conducted on carcinogenesis, mutagenesis, or impairment of fertililty with Octagam 5% liquid.
Repeated-dose toxicity studies and the genotoxic studies gave no evidence of carcinogenic properties of TNBP and Octoxynol [ 13 ], [ 14 ].
The results of in vitro and in vivo genotoxicity studies for TNBP and Octoxynol were negative. The results of studies on the embryotoxic and teratogenic properties of TNBP and Octoxynol in rats and rabbits at a wide range of i.v. doses were also negative.
Animal Toxicology and/or Pharmacology
No studies were conducted on non-clinical pharmacology, toxicology, local tolerance or pharmacokinetics with Octagam 5% liquid.
A variety of single-dose toxicity studies were performed for TNBP and Octoxynol alone or in combination. The lowest toxic dose of TNBP + Octoxynol (1+5) was 10,000 mcg/kg BM in rats after intravenous administration. Studies on 13-week toxicity were performed for combinations of TNBP + Octoxynol in a broad dose range intravenously in dogs and rats. In these studies the lowest toxic dose for rats was local 60mcg TNBP/kg +300mcg Octoxynol/kg BM i.v. (concentration: 0.0006% and 0.003%, respectively) and systemic 300mcg TNBP/kg + 1,500mcg Octoxynol/kg BM i.v. (concentration: 0.003% and 0.015%, respectively). The lowest toxic dose for dogs was local 50 mcg TNBP/kg + 250mcg Octoxynol/kg BM i.v. (concentration: 0.005% and 0.025%, respectively) and systemic 500mcg TNBP/kg + 2,500mcg Octoxynol/kg BM i.v. (concentration: 0.05% and 0.25%, respectively).
Local tolerance of TNBP and Octoxynol was evaluated from the experiments on repeat-dose toxicity (rats, dogs) and on developmental toxicity (rats, rabbits). In these animal studies the lowest dose exerting local adverse reactions was 50 + 250 mcg/kg BM (TNBP + Octoxynol; daily injections) in dogs. At this dose 4 out of 6 dogs were affected starting in week 7 of treatment.
A pharmacokinetic study was carried out in rats given 300 mcg of TNBP/kg and 1,500 mcg Octoxynol/kg BM i.v. The plasma half-life for TNBP was approximately 20 minutes. Octoxynol was not detected.
-
CLINICAL STUDIES
In an open-label, multicenter study, 46 patients (including 10 patients between the ages of 6 and 12, and one 15 years old) with primary humoral immunodeficiency (PI) received Octagam 5% liquid individualized doses of 300 - 600 mg/kg every 3 or 4 weeks for 12 months. Six patients discontinued the study prematurely.
Eligible patients had to meet the following key inclusion criteria: aged 3 years or older; had a PI that had as a significant component hypogammaglobulinemia or antibody deficiency; had been receiving IGIV replacement therapy at a steady dose for at least 3 months prior to study entry and had maintained a trough level of at least 320 mg/dL above baseline serum IgG levels.
Patients were excluded if they had a history of severe reactions to blood or any blood-derived product; if they had a selective IgA deficiency or demonstrable antibodies to IgA; if they received blood or any blood product or derivative other than a commercially available IGIV within 3 months prior to study entry; if they had hepatic function abnormalities or a positive direct Coombs test at screening; if they had a pre-existing renal impairment, a history of drug or alcohol abuse in the previous 12 months or acquired medical condition known to cause secondary immune deficiency; if they were receiving long-term daily treatment with steroids at a dose of at least 1 mg/kg/day; if they had a requirement for pre-medication for IGIV infusion other than aspirin, acetaminophen, or other non-steroidal anti-inflammatory drug, or antihistamine; and if the received immunosuppressive or immunomodulatory drugs.
The primary efficacy endpoint was the number of episodes of serious infections/patient/year. Serious infection included pneumonia, bacteremia or sepsis, osteomyelitis/septic arthritis, visceral abscesses or bacterial or viral meningitis. Secondary efficacy variables were: the number of days of work/school missed; the number and days of hospitalizations; the number of visits to physicians for acute problems and/or visits to hospital emergency rooms; and the number of other infections documented by radiograph and fever.
For the primary endpoint, which was the number of episodes of serious infections, the observed rate was 0.1 infections per patient per year (5 infections over 43.5 patient-years).
Table 10: Summary of Secondary Efficacy Variables
Variable Subjects N Subjects % Total Days or Visits Total Subject Years Days or Visits/Subj./YearEstimate Work/School Days Missed
30
65
241
43.5
5.5
Days in Hospital
4
9
16
43.5
0.4
Visits to Physician/ER
27
59
92
43.5
2.1
-
REFERENCES
- Duhem, C., Dicato, M. A. and Ries, F. Side-effects of intravenous immune globulins. Clin. Exp. Immunol. 97 Suppl 1, 79-83 (1994).
- Kannan, S., Rowland, C. H., Hockings, G. I. and Tauchmann, P. M. Intragam can interfere with blood glucose monitoring. Med. J. Aust. 180, 251-252 (2004).
- Steinberger, B. A., Ford, S. M., Coleman, T. A. Intravenous Immunoglobulin Therapy Results in Post-infusional Hyperproteinemia, Increased Serum Viscosity, and Pseudohyponatremia. Am J Hematol 73:97-100 (2003)
- Dalakas, M. C. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 44, 223-226 (1994).
- Wolberg, A. S., Kon, R. H., Monroe, D. M. and Hoffman, M. Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Am. J Hematol. 65, 30-34 (2000).
- Go RS, Call TG: Deep venous thrombosis of the arm after intravenous immunoglobulin infusion: case report and literature review of intravenous immunoglobulin-related thrombotic complications. Mayo Clin Proc75:83-85 (2000)
- Sekul, E. A., Cupler, E. J. and Dalakas, M. C. Aseptic meningitis associated with high-dose intravenous immunoglobulin therapy: frequency and risk factors. Ann Intern. Med. 121, 259-262 (1994).
- Pierce LR, Jain N: Risks associated with the use of intravenous immunoglobulin. Transfus Med Rev 17:241-251 (2003)
- Kessary-Shoham H., Levy, Y., Shoenfeld, Y., Lorber, M. and Gershon, H. In vivo administration of intravenous immunoglobulin (IVIg) can lead to enhanced erythrocyte sequestration. Journal of Autoimmunity. 13, 129-135 (1999).
- Rizk, A., Gorson, K. C., Kenney, L. and Weinstein, R. Transfusion-related acute lung injury after the infusion of IVIG. Transfusion 41, 264-268 (2001).
- Koleba T, Ensom MH: Pharmacokinetics of intravenous immunoglobulin: a systematic review. Pharmacotherapy 26:813-827 (2006)
- Lever, A. M., Yap, P. L., Cuthbertson, B., Wootton, R. and Webster, A. D. Increased half-life of gammaglobulin after prolonged intravenous replacement therapy. Clin Exp. Immunol 67, 441-446 (1987).
- Auletta, CS., Kotkoskie, LA., Saulog, T., Richter, WR. A dietary oncogenicity study of tributyl phosphate in the CD-1 mouse. Toxicology 128, 135-141 (1998a).
- Auletta CS., Weiner ML., Richter WR. A dietary toxicity/oncogenicity study of tributyl phosphate in the rat. Toxicology 128: 125-134 (1998b).
Octagam 5% liquid is supplied in 1.0 g, 2.5 g, 5 g, 10 g or 25 g single use bottles.
NDC Number NDC Number Size Grams Protein Octapharma Pharmazeutika Produktionsges.m.b.H
Octapharma AB
67467 – 843 - 01
68209 – 843 - 01
20 ml
1.0
67467 – 843 – 02
68209 – 843 – 02
50 ml
2.5
67467 – 843 – 03
68209 – 843 – 03
100 ml
5.0
67467 – 843 – 04
68209 – 843 – 04
200 ml
10.0
67467 – 843 – 05
500 ml
25.0
Octagam 5% liquid is not supplied with an infusion set. If a filtered infusion set is used (not mandatory), the filter size must be 0.2 – 200 microns.
Components used in the packaging of Octagam 5% liquid are latex-free.
Octagam 5% liquid may be stored for 24 months at +2°C to + 25°C (36°F to 77°F) from the date of manufacture.
Do not use after expiration date.
Do not freeze. Frozen product should not be used.
Any unused product or waste material should be disposed of in accordance with local requirements.
-
PATIENT COUNSELING INFORMATION
Information for Patients
Inform patients of the early signs of hypersensitivity reactions including hives, generalised urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If allergic symptoms occur, patients should contact their physicians immediately.
Inform patients to also immediately report the following to their physician:
signs and symptoms of renal failures, such as decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath
signs and symptoms of aseptic meningitis, such as headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting
signs and symptoms of hemolysis, such as fatigue, increased heart rate, yellowing of the skin or eyes, and dark-colored urine
signs and symptoms of TRALI, such as severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, fever. TRALI typically occurs within 1 to 6 hours following transfusion
Inform patients that Octagam 5% liquid is made from human plasma and may contain infectious agents that can cause disease (e.g., viruses, and, theoretically, the CJD agent). Inform patients that the risk Octagam 5% liquid may transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing the donated plasma for certain virus infections and by inactivating and/or removing certain viruses during manufacturing.
Inform patients that administration of IgG may interfere with the response to live viral vaccines such as measles, mumps and rubella. Inform patients to notify their immunizing physician of therapy with Octagam 5% liquid.
Octapharma USA Inc.Hoboken, NJ 07030
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL
Immune Globulin Intravenous (Human), 5%
Octapharma Pharmazeutika Produktionsges.m.b.H
20 mL
NDC: 67467-843-01

100mL
NDC: 67467-843-03

-
INGREDIENTS AND APPEARANCE
OCTAGAM IMMUNE GLOBULIN (HUMAN)
immune globulin solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67467-843 Route of Administration Intravenous Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 50 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67467-843-05 500 mL in 1 BOTTLE, GLASS 2 NDC: 67467-843-04 200 mL in 1 BOTTLE, GLASS 3 NDC: 67467-843-03 100 mL in 1 BOTTLE, GLASS 4 NDC: 67467-843-02 50 mL in 1 BOTTLE, GLASS 5 NDC: 67467-843-01 20 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125062 05/21/2004 Labeler - Octapharma Pharmazeutika Produktionsgesellschaft m.b.H. (301119178)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.