Mederma Scar Cream by HRA Pharma America, Inc. / Merz Pharma GmbH & Co KGaA Drug Facts
Mederma Scar Cream by
Drug Labeling and Warnings
Mederma Scar Cream by is a Otc medication manufactured, distributed, or labeled by HRA Pharma America, Inc., Merz Pharma GmbH & Co KGaA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MEDERMA SCAR CREAM- avobenzone, octocrylene, and oxybenzone cream
HRA Pharma America, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
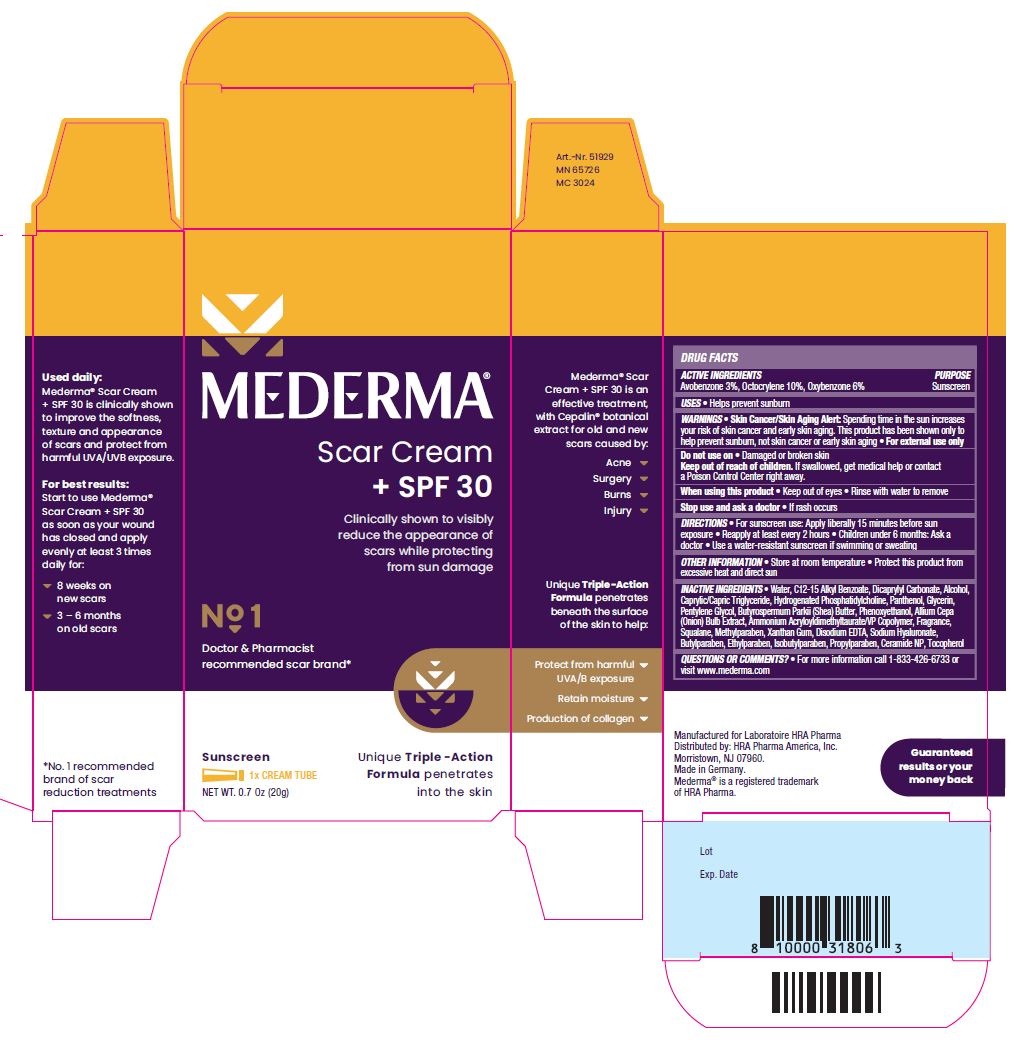
Drug Facts
WARNINGS Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging. For external use only
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS For sunscreen use: Apply liberally 15 minutes before sun exposure Reapply at least every 2 hours Children under 6 months: Ask a doctor Use a water-resistant sunscreen if swimming or sweating
INACTIVE INGREDIENTS Water, C12-15 Alkyl Benzoate, Dicaprylyl Carbonate, Alcohol, Caprylic/Capric Triglyceride, Hydrogenated Phosphatidylcholine, Panthenol, Glycerin, Pentylene Glycol, Butyrospermum Parkii (Shea) Butter, Phenoxyethanol, Allium Cepa (Onion) Bulb Extract, Ammonium Acryloyldimethyltaurate/VP Copolymer, Fragrance, Squalane, Methylparaben, Xanthan Gum, Disodium EDTA, Sodium Hyaluronate, Butylparaben, Ethylparaben, Isobutylparaben, Propylparaben, Ceramide NP, Tocopherol
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
MEDERMA®
Scar Cream + SPF 30
Clinically shown to visibly reduce the appearance of scars while protecting from sun damage
Unique Triple-Action Formula penetrates into the skin
No 1 Doctor & Pharmacist recommended scar brand*
Sunscreen
1 x CREAM TUBE
NET WT. 0.7 0z (20g)
MEDERMA® Scar Cream + SPF 30 is an effective treatment, with Cepalin® botanical extract for old and new scars caused by:
- Acne
- Surgery
- Burns
- Injury
Unique Triple-Action Formula penetrates beneath the surface of the skin to help:
- Protect from harmful UVA/B exposure
- Retain moisture
- Production of collagen
Used daily: Mederma® Scar Cream + SPF 30 is clinically shown to improve the softness, texture and appearance of scars and protect from harmful UVA/UVB exposure.
For best results: Start to use Mederma® Scar Cream + SPF 30 as soon as your wound has closed and apply evenly at least 3 times daily for:
- 8 weeks on new scars
- 3-6 months on old scars
Guaranteed results or your money back
*No. 1 recommended brand of scar reduction treatments
Manufactured for Laboratoire HRA Pharma
Distributed by: HRA Pharma America, Inc.
Morristown, NJ 07960.
Made in Germany.
Mederma® is a registered trademark of HRA Pharma
Lot
Exp. Date
| MEDERMA SCAR CREAM
avobenzone, octocrylene, and oxybenzone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - HRA Pharma America, Inc. (081160441) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Merz Pharma GmbH & Co KGaA | 342543179 | manufacture(73302-200) | |