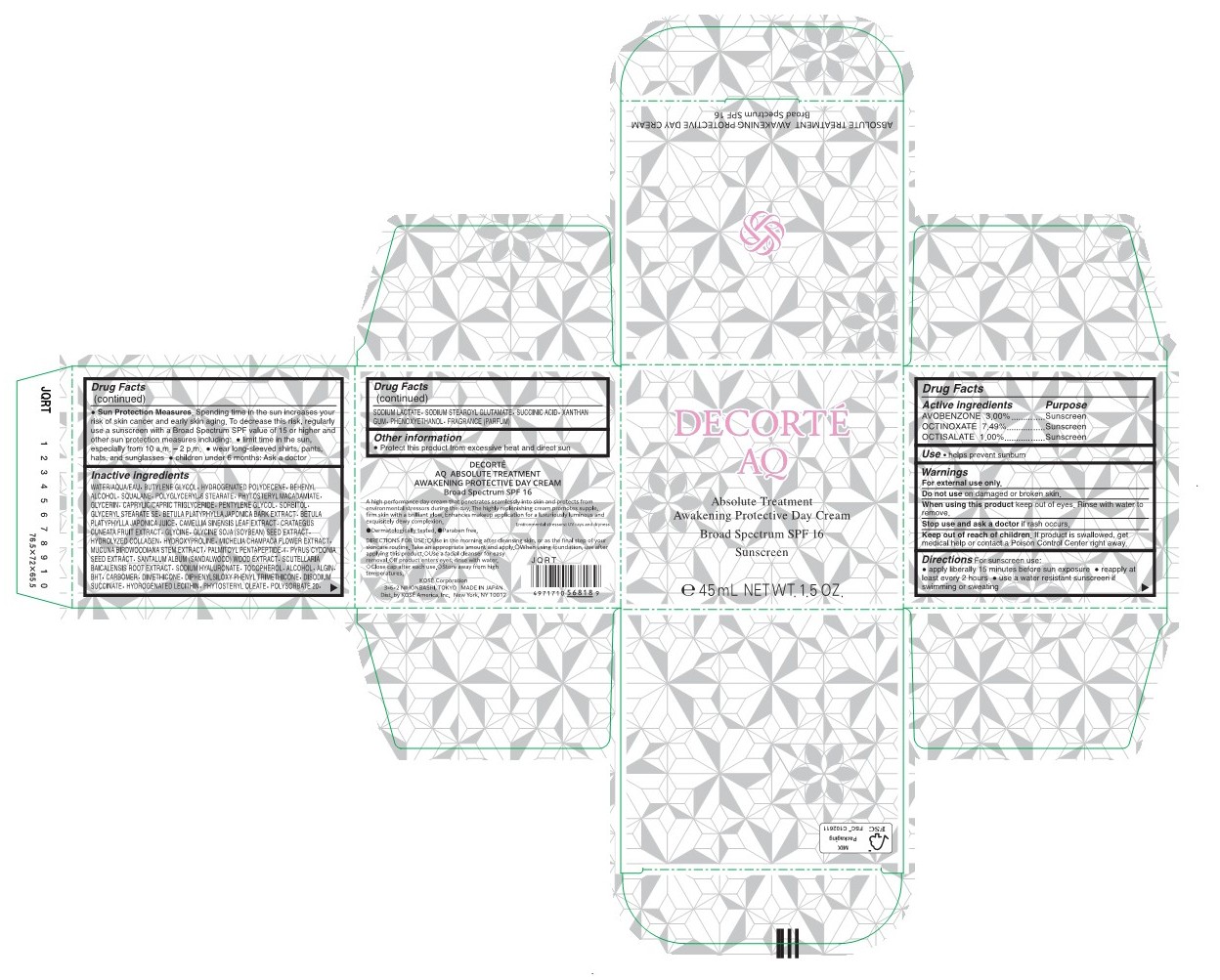
DECORTE AQ ABSOLUTE TREATMENT AWAKENING PROTECTIVE DAY- avobenzone, octinoxate, octisalate cream
DECORTE AQ ABSOLUTE TREATMENT AWAKENING PROTECTIVE DAY by
Drug Labeling and Warnings
DECORTE AQ ABSOLUTE TREATMENT AWAKENING PROTECTIVE DAY by is a Otc medication manufactured, distributed, or labeled by KOSÉ America, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
-
USES
- Helps Prevent Sunburn
DIRECTIONS FOR USE:
- Use in the morning after cleansing skin, or as the final step of your skincare routine. Take an appropriate amount and apply.
- When using foundation, use after applying this product.
- Use a facial cleanser for easy removal.
- lf product enters eyes, rinse with water.
- Close cap after each use.
- Store away from high temperatures.
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
For sunscreen use:
apply liberally 15 minutes before sun exposure
reapply at least every 2 hours
use a water resistant sunscreen if swimming or sweating
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
children under 6 months: Ask a doctor -
INACTIVE INGREDIENT
Water/AQUA/EAU, Butylene Glycol, Hydrogenated Polydecene, Behenyl Alcohol, Squalane, Polyglyceryl-6 Stearate, Phytosteryl Macadamiate, Glycerin, Caprylic/Capric Triglyceride, Pentylene Glycol, Sorbitol, Glyceryl Stearate SE, Betula Platyphylla Japonica Bark Extract, Betula Platyphylla Japonika Juice, Camellia Sinensis Leaf Extract, Crataegus Cuneata Fruit Extract, Glycine, Glycine Soja (Soybean) Seed Extract, Hydrolyzed Collagen, Hydroxyproline, Michelia Champaca Flower Extract, Mucuna Birdwoodiana Stem Extract, Palmitoyl Pentapeptide-4, Pyrus Cydonia Seed Extract, Santalum Album (Sandalwood) Wood Extract, Scutellaria Baicalensis Root Extract, Sodium Hyaluronate, Tocopherol, Alcohol, Algin, BHT, Carbomer, Dimethicone, Diphenylsiloxy Phenyl Trimethicone, Disodium Succinate, Hydrogenated Lecithin, Phytosteryloleate, Polysorbate 20, Sodium Lactate, Sodium Stearoyl Glutamate, Succinic Acid, Xanthan Gum, Phenoxyethanol, Fragrance (Parfum)
- Other information
- PRINCIPAL DISPLAY PANEL - 45 mL CYLINDER
-
INGREDIENTS AND APPEARANCE
DECORTE AQ ABSOLUTE TREATMENT AWAKENING PROTECTIVE DAY
avobenzone, octinoxate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 66820-0500 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 74.945 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYDROGENATED POLYDECENE TYPE I (UNII: U333RI6EB7) ALCOHOL (UNII: 3K9958V90M) SQUALANE (UNII: GW89575KF9) DOCOSANOL (UNII: 9G1OE216XY) POLYGLYCERYL-6 STEARATE (UNII: ETY9Q81E2T) PHYTOSTERYL MACADAMIATE (UNII: 233VSF903M) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PENTYLENE GLYCOL (UNII: 50C1307PZG) SORBITOL (UNII: 506T60A25R) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) BETULA PLATYPHYLLA RESIN (UNII: 1B33PG6N4K) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) XANTHAN GUM (UNII: TTV12P4NEE) GLYCINE (UNII: TE7660XO1C) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SODIUM ALGINATE (UNII: C269C4G2ZQ) HYDROLYZED ORYZANOL OLEATES (UNII: CZ7DW0L6FX) HYDROXYPROLINE (UNII: RMB44WO89X) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) MUCUNA BIRDWOODIANA STEM (UNII: 6W7VXE8RS6) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CYDONIA OBLONGA SEED (UNII: JXU526QH1V) CRATAEGUS CUNEATA FRUIT (UNII: 98TEA9T639) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MARINE NON-GELLING GELATIN (UNII: JSM64OJO9B) SODIUM LACTATE (UNII: TU7HW0W0QT) MICHELIA CHAMPACA FLOWER (UNII: VOT99IIA37) GREEN TEA LEAF (UNII: W2ZU1RY8B0) POLYSORBATE 20 (UNII: 7T1F30V5YH) SUCCINIC ACID (UNII: AB6MNQ6J6L) SOYBEAN (UNII: L7HT8F1ZOD) BETULA PLATYPHYLLA BARK (UNII: ZF70YKN0YO) SANDALWOOD (UNII: 3641YW25N2) PALMITOYL PENTAPEPTIDE-4 (UNII: KK181SM5JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66820-0500-1 1 in 1 CARTON 05/16/2024 1 45 mL in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/16/2024 Labeler - KOSÉ America, Inc. (080407621)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.