PREZISTA- darunavir tablet, film coated PREZISTA- darunavir suspension
PREZISTA by
Drug Labeling and Warnings
PREZISTA by is a Prescription medication manufactured, distributed, or labeled by Janssen Products LP, JANSSEN ORTHO LLC, Cilag AG, Janssen Pharmaceutical Sciences Unlimited Company, Janssen Pharmaceuticals, Inc, Janssen Pharmaceutica NV, Janssen Cilag SpA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PREZISTA safely and effectively. See Full Prescribing Information for PREZISTA.
PREZISTA (darunavir) oral suspension
PREZISTA (darunavir) tablet, for oral use
Initial U.S. Approval: 2006RECENT MAJOR CHANGES
INDICATIONS AND USAGE
PREZISTA is a human immunodeficiency virus (HIV-1) protease inhibitor indicated for the treatment of HIV-1 infection in adult and pediatric patients 3 years of age and older. PREZISTA must be co-administered with ritonavir (PREZISTA/ritonavir) and with other antiretroviral agents. (1)
DOSAGE AND ADMINISTRATION
- Testing:
- In treatment-experienced patients, treatment history genotypic and/or phenotypic testing is recommended prior to initiation of therapy with PREZISTA/ritonavir to assess drug susceptibility of the HIV-1 virus (2.1, 12.4)
- Monitor serum liver chemistry tests before and during therapy with PREZISTA/ritonavir. (2.1, 2.2, 5.2)
- Treatment-naïve adult patients and treatment-experienced adult patients with no darunavir resistance associated substitutions: 800 mg (one 800 mg tablet) taken with ritonavir 100 mg once daily and with food. (2.3)
- Treatment-experienced adult patients with at least one darunavir resistance associated substitution: 600 mg (one 600 mg tablet) taken with ritonavir 100 mg twice daily and with food. (2.3)
- Pregnant patients: 600 mg (one 600 mg tablet) taken with ritonavir 100 mg twice daily and with food. (2.4)
- Pediatric patients (3 to less than 18 years of age and weighing at least 10 kg): dosage of PREZISTA and ritonavir is based on body weight and should not exceed the adult dose. PREZISTA should be taken with ritonavir and with food. (2.5)
- PREZISTA/ritonavir is not recommended for use in patients with severe hepatic impairment. (2.6)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Co-administration of PREZISTA/ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). (4)
WARNINGS AND PRECAUTIONS
- Drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) has been reported with PREZISTA/ritonavir. Monitor liver function before and during therapy, especially in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases. Post-marketing cases of liver injury, including some fatalities, have been reported. (5.2)
- Skin reactions ranging from mild to severe, including Stevens-Johnson Syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms and acute generalized exanthematous pustulosis, have been reported. Discontinue treatment if severe reaction develops. (5.3)
- Use with caution in patients with a known sulfonamide allergy. (5.4)
- Patients may develop new onset diabetes mellitus or hyperglycemia. Initiation or dose adjustments of insulin or oral hypoglycemic agents may be required. (5.6)
- Patients may develop redistribution/accumulation of body fat or immune reconstitution syndrome. (5.7, 5.8)
- Patients with hemophilia may develop increased bleeding events. (5.9)
- PREZISTA/ritonavir is not recommended in pediatric patients below 3 years of age in view of toxicity and mortality observed in juvenile rats dosed with darunavir up to days 23 to 26 of age. (5.10)
ADVERSE REACTIONS
- The most common clinical adverse drug reactions to PREZISTA/ritonavir (incidence greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, nausea, rash, headache, abdominal pain and vomiting. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Products, LP at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: Total darunavir exposures were generally lower during pregnancy compared to postpartum period. The reduction in darunavir exposures during pregnancy were greater for once daily dosing compared to the twice daily dosing regimen. (8.1, 12.3)
- Lactation: Women infected with HIV should be instructed not to breastfeed due to the potential for HIV transmission. (8.2)
- Pediatrics: Not recommended for patients less than 3 years of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2019
- Testing:
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of PREZISTA/ritonavir
2.2 Monitoring During Treatment with PREZISTA/ritonavir
2.3 Recommended Dosage in Adult Patients
2.4 Recommended Dosage During Pregnancy
2.5 Recommended Dosage in Pediatric Patients (age 3 to less than 18 years)
2.6 Not Recommended in Patients with Severe Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Importance of Co-administration with Ritonavir
5.2 Hepatotoxicity
5.3 Severe Skin Reactions
5.4 Sulfa Allergy
5.5 Risk of Serious Adverse Reactions due to Drug Interactions
5.6 Diabetes Mellitus/Hyperglycemia
5.7 Fat Redistribution
5.8 Immune Reconstitution Syndrome
5.9 Hemophilia
5.10 Not Recommended in Pediatric Patients Below 3 Years of Age
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Potential for PREZISTA/ritonavir to Affect Other Drugs
7.2 Potential for Other Drugs to Affect Darunavir
7.3 Established and Other Potentially Significant Drug Interactions
7.4 Drugs without Clinically Significant Interactions with PREZISTA
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Description of Adult Clinical Trials
14.2 Treatment-Naïve Adult Subjects
14.3 Treatment-Experienced Adult Subjects
14.4 Pediatric Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
PREZISTA®, co-administered with ritonavir (PREZISTA/ritonavir), in combination with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus (HIV-1) infection in adult and pediatric patients 3 years of age and older [see Use in Specific Populations (8.4) and Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of PREZISTA/ritonavir
In treatment-experienced patients, treatment history, genotypic and/or phenotypic testing is recommended to assess drug susceptibility of the HIV-1 virus [see Microbiology (12.4)]. Refer to Dosage and Administration (2.3), (2.4) and (2.5) for dosing recommendations.
Appropriate laboratory testing such as serum liver biochemistries should be conducted prior to initiating therapy with PREZISTA/ritonavir [see Warnings and Precautions (5.2)].
2.2 Monitoring During Treatment with PREZISTA/ritonavir
Patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases should be monitored for elevation in serum liver biochemistries, especially during the first several months of PREZISTA/ritonavir treatment [see Warnings and Precautions (5.2)].
2.3 Recommended Dosage in Adult Patients
PREZISTA must be co-administered with ritonavir to exert its therapeutic effect. Failure to correctly co-administer PREZISTA with ritonavir will result in plasma levels of darunavir that will be insufficient to achieve the desired antiviral effect and will alter some drug interactions.
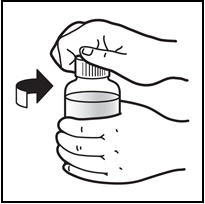
Patients who have difficulty swallowing PREZISTA tablets can use the 100 mg per mL PREZISTA oral suspension.
Treatment-Naïve Adult Patients
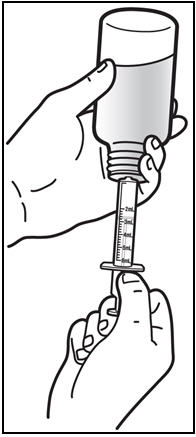
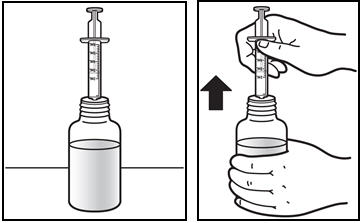
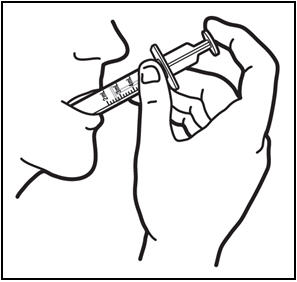
The recommended oral dose of PREZISTA is 800 mg (one 800 mg tablet or 8 mL of the oral suspension) taken with ritonavir 100 mg (one 100 mg tablet or capsule or 1.25 mL of a 80 mg per mL ritonavir oral solution) once daily and with food. An 8 mL PREZISTA dose should be taken as two 4 mL administrations with the included oral dosing syringe.
Treatment-Experienced Adult Patients
The recommended oral dosage for treatment-experienced adult patients is summarized in Table 1.
Baseline genotypic testing is recommended for dose selection. However, when genotypic testing is not feasible, PREZISTA 600 mg taken with ritonavir 100 mg twice daily is recommended.
Table 1: Recommended PREZISTA/ritonavir Dosage in Treatment-Experienced Adult Patients Formulation and Recommended Dosing Baseline Resistance PREZISTA tablets with ritonavir tablets or capsule PREZISTA oral suspension (100 mg/mL) with ritonavir oral solution (80 mg/mL) - * V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V and L89V
- † An 8 mL darunavir dose should be taken as two 4 mL administrations with the included oral dosing syringe
With no darunavir resistance associated substitutions* One 800 mg PREZISTA tablet with one 100 mg ritonavir tablet/capsule, taken once daily with food 8 mL† PREZISTA oral suspension with 1.25 mL ritonavir oral solution, taken once daily with food With at least one darunavir resistance associated substitutions*, or with no baseline resistance information One 600 mg PREZISTA tablet with one 100 mg ritonavir tablet/capsule, taken twice daily with food 6 mL PREZISTA oral suspension with 1.25 mL ritonavir oral solution, taken twice daily with food 2.4 Recommended Dosage During Pregnancy
The recommended dosage in pregnant patients is PREZISTA 600 mg taken with ritonavir 100 mg twice daily with food.
PREZISTA 800 mg taken with ritonavir 100 mg once daily should only be considered in certain pregnant patients who are already on a stable PREZISTA 800 mg with ritonavir 100 mg once daily regimen prior to pregnancy, are virologically suppressed (HIV-1 RNA less than 50 copies per mL), and in whom a change to twice daily PREZISTA 600 mg with ritonavir 100 mg may compromise tolerability or compliance.
2.5 Recommended Dosage in Pediatric Patients (age 3 to less than 18 years)
Healthcare professionals should pay special attention to accurate dose selection of PREZISTA, transcription of the medication order, dispensing information and dosing instruction to minimize risk for medication errors, overdose, and underdose.
Prescribers should select the appropriate dose of PREZISTA/ritonavir for each individual child based on body weight (kg) and should not exceed the recommended dose for adults.
Before prescribing PREZISTA, children weighing greater than or equal to 15 kg should be assessed for the ability to swallow tablets. If a child is unable to reliably swallow a tablet, the use of PREZISTA oral suspension should be considered.
The recommended dose of PREZISTA/ritonavir for pediatric patients (3 to less than 18 years of age and weighing at least 10 kg is based on body weight (see Tables 2, 3, 4, and 5) and should not exceed the recommended adult dose. PREZISTA should be taken with ritonavir and with food.
The recommendations for the PREZISTA/ritonavir dosage regimens were based on pediatric clinical trial data and population pharmacokinetic modeling and simulation [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)].
Dosing Recommendations for Treatment-Naïve Pediatric Patients or Antiretroviral Treatment-Experienced Pediatric Patients with No Darunavir Resistance Associated Substitutions
Pediatric Patients Weighing At Least 10 kg but Less than 15 kg
The weight-based dose in antiretroviral treatment-naïve pediatric patients or antiretroviral treatment-experienced pediatric patients with no darunavir resistance associated substitutions is PREZISTA 35 mg/kg once daily with ritonavir 7 mg/kg once daily using the following table:
Table 2: Recommended Dose for Pediatric Patients Weighing 10 kg to Less Than 15 kg Who are Treatment-Naïve or Treatment-Experienced with No Darunavir Resistance Associated Substitutions* Body weight (kg) Formulation: PREZISTA oral suspension (100 mg/mL) and ritonavir oral solution (80 mg/mL) Dose: once daily with food - * darunavir resistance associated substitutions: V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V and L89V
- † The 350 mg, 385 mg, 455 mg and 490 mg darunavir dose for the specified weight groups were rounded up for suspension dosing convenience to 3.6 mL, 4 mL, 4.6 mL and 5 mL, respectively.
Greater than or equal to 10 kg to less than 11 kg
PREZISTA 3.6 mL† (350 mg) with ritonavir 0.8 mL (64 mg) Greater than or equal to 11 kg to less than 12 kg
PREZISTA 4 mL† (385 mg) with ritonavir 0.8 mL (64 mg) Greater than or equal to 12 kg to less than 13 kg
PREZISTA 4.2 mL (420 mg) with ritonavir 1 mL (80 mg) Greater than or equal to 13 kg to less than 14 kg
PREZISTA 4.6 mL† (455 mg) with ritonavir 1 mL (80 mg) Greater than or equal to 14 kg to less than 15 kg
PREZISTA 5 mL† (490 mg) with ritonavir 1.2 mL (96 mg) Pediatric Patients Weighing At Least 15 kg
Pediatric patients weighing at least 15 kg can be dosed with PREZISTA oral tablet(s) or suspension using the following table:
Table 3: Recommended Dose for Pediatric Patients Weighing At Least 15 kg Who are Treatment-Naïve or Treatment-Experienced with No Darunavir Resistance Associated Substitutions* Body weight (kg) Formulation: PREZISTA tablet(s) and ritonavir capsules or tablets (100 mg) Formulation: PREZISTA oral suspension (100 mg/mL) and ritonavir oral solution (80 mg/mL) Dose: once daily with food Dose: once daily with food - * darunavir resistance associated substitutions: V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V and L89V
- † The 675 mg dose using darunavir tablets for this weight group is rounded up to 6.8 mL for suspension dosing convenience.
- ‡ The 6.8 mL and 8 mL darunavir dose should be taken as two (3.4 mL or 4 mL respectively) administrations with the included oral dosing syringe
Greater than or equal to 15 kg to less than 30 kg PREZISTA 600 mg with ritonavir 100 mg PREZISTA 6 mL (600 mg) with ritonavir 1.25 mL (100 mg) Greater than or equal to 30 kg to less than 40 kg PREZISTA 675 mg with ritonavir 100 mg PREZISTA 6.8 mL†‡ (675 mg) with ritonavir 1.25 mL (100 mg) Greater than or equal to 40 kg PREZISTA 800 mg with ritonavir 100 mg PREZISTA 8 mL‡ (800 mg) with ritonavir 1.25 mL (100 mg) Dosing Recommendations for Treatment-Experienced Pediatric Patients with At Least One Darunavir Resistance Associated Substitutions
Pediatric Patients Weighing At Least 10 kg but Less than 15 kg
The weight-based dose in antiretroviral treatment-experienced pediatric patients with at least one darunavir resistance associated substitution is PREZISTA 20 mg/kg twice daily with ritonavir 3 mg/kg twice daily using the following table:
Table 4: Recommended Dose for Pediatric Patients Weighing 10 kg to Less Than 15 kg Who are Treatment-Experienced with At Least One Darunavir Resistance Associated Substitution* Body weight (kg) Formulation: PREZISTA oral suspension (100 mg/mL) and ritonavir oral solution (80 mg/mL) Dose: twice daily with food - * darunavir resistance associated substitutions: V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V and L89V
Greater than or equal to 10 kg to less than 11 kg PREZISTA 2 mL (200 mg) with ritonavir 0.4 mL (32 mg) Greater than or equal to 11 kg to less than 12 kg PREZISTA 2.2 mL (220 mg) with ritonavir 0.4 mL (32 mg) Greater than or equal to 12 kg to less than 13 kg PREZISTA 2.4 mL (240 mg) with ritonavir 0.5 mL (40 mg) Greater than or equal to 13 kg to less than 14 kg PREZISTA 2.6 mL (260 mg) with ritonavir 0.5 mL (40 mg) Greater than or equal to 14 kg to less than 15 kg PREZISTA 2.8 mL (280 mg) with ritonavir 0.6 mL (48 mg) Pediatric Patients Weighing At Least 15 kg
Pediatric patients weighing at least 15 kg can be dosed with PREZISTA oral tablet(s) or suspension using the following table:
Table 5: Recommended Dose for Pediatric Patients Weighing At Least 15 kg Who are Treatment-Experienced with At Least One Darunavir Resistance Associated Substitution* Body weight (kg) Formulation: PREZISTA tablet(s) and ritonavir tablets, capsules (100 mg) or oral solution (80 mg/mL) Formulation: PREZISTA oral suspension (100 mg/mL) and ritonavir oral solution (80 mg/mL) Dose: twice daily with food Dose: twice daily with food - * darunavir resistance associated substitutions: V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V and L89V
- † The 375 mg and 450 mg dose using darunavir tablets for this weight group is rounded up to 3.8 mL and 4.6 mL for suspension dosing convenience.
Greater than or equal to 15 kg to less than 30 kg PREZISTA 375 mg with ritonavir 0.6 mL (48 mg) PREZISTA 3.8 mL (375 mg)† with ritonavir 0.6 mL (48 mg) Greater than or equal to 30 kg to less than 40 kg PREZISTA 450 mg with ritonavir 0.75 mL (60 mg) PREZISTA 4.6 mL (450 mg)† with ritonavir 0.75 mL (60 mg) Greater than or equal to 40 kg PREZISTA 600 mg with ritonavir 100 mg PREZISTA 6 mL (600 mg) with ritonavir 1.25 mL (100 mg) The use of PREZISTA/ritonavir in pediatric patients below 3 years of age is not recommended [see Warnings and Precautions (5.10) and Use in Specific Populations (8.4)].
2.6 Not Recommended in Patients with Severe Hepatic Impairment
No dosage adjustment is required in patients with mild or moderate hepatic impairment. No data are available regarding the use of PREZISTA/ritonavir when co-administered to subjects with severe hepatic impairment; therefore, PREZISTA/ritonavir is not recommended for use in patients with severe hepatic impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
PREZISTA Oral Suspension
PREZISTA 100 mg per mL is supplied as a white to off-white opaque suspension for oral use, containing darunavir ethanolate equivalent to 100 mg of darunavir per mL of suspension.
PREZISTA Tablets
- 75 mg: white, caplet-shaped, film-coated tablets containing darunavir ethanolate equivalent to 75 mg of darunavir. Each tablet is debossed with "75" on one side and "TMC" on the other side.
- 150 mg: white, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 150 mg of darunavir. Each tablet is debossed with "150" on one side and "TMC" on the other side.
- 600 mg: orange, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 600 mg of darunavir. Each tablet is debossed with "600MG" on one side and "TMC" on the other side.
- 800 mg: dark red, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 800 mg of darunavir. Each tablet is debossed with "800" on one side and "T" on the other side.
-
4 CONTRAINDICATIONS
Co-administration of PREZISTA/ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). These drugs and other contraindicated drugs (which may lead to reduced efficacy of darunavir) are listed below [see Drug Interactions (7.3)]. Due to the need for co-administration of PREZISTA with ritonavir, please refer to ritonavir prescribing information for a description of ritonavir contraindications.
- Alpha 1-adrenoreceptor antagonist: alfuzosin
- Anti-gout: colchicine, in patients with renal and/or hepatic impairment
- Antimycobacterial: rifampin
- Antipsychotics: lurasidone, pimozide
- Cardiac Disorders: dronedarone, ivabradine, ranolazine
- Ergot derivatives, e.g. dihydroergotamine, ergotamine, methylergonovine
- GI motility agent: cisapride
- Herbal product: St. John's wort (Hypericum perforatum)
- Hepatitis C direct acting antiviral: elbasvir/grazoprevir
- Lipid modifying agents: lomitapide, lovastatin, simvastatin
- Opioid Antagonist: naloxegol
- PDE-5 inhibitor: sildenafil when used for treatment of pulmonary arterial hypertension
- Sedatives/hypnotics: orally administered midazolam, triazolam
-
5 WARNINGS AND PRECAUTIONS
5.1 Importance of Co-administration with Ritonavir
PREZISTA must be co-administered with ritonavir and food to achieve the desired antiviral effect. Failure to administer PREZISTA with ritonavir and food may result in a loss of efficacy of darunavir.
Please refer to ritonavir prescribing information for additional information on precautionary measures.
5.2 Hepatotoxicity
Drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) has been reported with PREZISTA/ritonavir. During the clinical development program (N=3063), hepatitis was reported in 0.5% of patients receiving combination therapy with PREZISTA/ritonavir. Patients with pre-existing liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities including severe hepatic adverse events.
Post-marketing cases of liver injury, including some fatalities, have been reported. These have generally occurred in patients with advanced HIV-1 disease taking multiple concomitant medications, having co-morbidities including hepatitis B or C co-infection, and/or developing immune reconstitution syndrome. A causal relationship with PREZISTA/ritonavir therapy has not been established.
Appropriate laboratory testing should be conducted prior to initiating therapy with PREZISTA/ritonavir and patients should be monitored during treatment. Increased AST/ALT monitoring should be considered in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases, especially during the first several months of PREZISTA/ritonavir treatment.
Evidence of new or worsening liver dysfunction (including clinically significant elevation of liver enzymes and/or symptoms such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) in patients on PREZISTA/ritonavir should prompt consideration of interruption or discontinuation of treatment.
5.3 Severe Skin Reactions
During the clinical development program (n=3063), severe skin reactions, accompanied by fever and/or elevations of transaminases in some cases, have been reported in 0.4% of subjects. Stevens-Johnson Syndrome was rarely (less than 0.1%) reported during the clinical development program. During post-marketing experience toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis have been reported. Discontinue PREZISTA/ritonavir immediately if signs or symptoms of severe skin reactions develop. These can include but are not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and/or eosinophilia.
Rash (all grades, regardless of causality) occurred in 10.3% of subjects treated with PREZISTA/ritonavir [also see Adverse Reactions (6)]. Rash was mostly mild-to-moderate, often occurring within the first four weeks of treatment and resolving with continued dosing. The discontinuation rate due to rash in subjects using PREZISTA/ritonavir was 0.5%.
Rash occurred more commonly in treatment-experienced subjects receiving regimens containing PREZISTA/ritonavir + raltegravir compared to subjects receiving PREZISTA/ritonavir without raltegravir or raltegravir without PREZISTA/ritonavir. However, rash that was considered drug related occurred at similar rates for all three groups. These rashes were mild to moderate in severity and did not limit therapy; there were no discontinuations due to rash.
5.4 Sulfa Allergy
Darunavir contains a sulfonamide moiety. PREZISTA should be used with caution in patients with a known sulfonamide allergy. In clinical studies with PREZISTA/ritonavir, the incidence and severity of rash were similar in subjects with or without a history of sulfonamide allergy.
5.5 Risk of Serious Adverse Reactions due to Drug Interactions
Initiation of PREZISTA/ritonavir, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving PREZISTA/ritonavir, may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of PREZISTA/ritonavir, respectively. These interactions may lead to:
- Clinically significant adverse reactions, potentially leading to severe, life threatening, or fatal events from greater exposures of concomitant medications.
- Clinically significant adverse reactions from greater exposures of PREZISTA/ritonavir.
- Loss of therapeutic effect of PREZISTA/ritonavir and possible development of resistance.
See Table 10 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during PREZISTA/ritonavir therapy; review concomitant medications during PREZISTA/ritonavir therapy; and monitor for the adverse reactions associated with the concomitant drugs [see Contraindications (4) and Drug Interactions (7)].
5.6 Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor (PI) therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued PI therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between PI therapy and these events have not been established.
5.7 Fat Redistribution
Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.8 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including PREZISTA. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of antiretroviral treatment.
5.9 Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with PIs. In some patients, additional factor VIII was given. In more than half of the reported cases, treatment with PIs was continued or reintroduced if treatment had been discontinued. A causal relationship between PI therapy and these episodes has not been established.
5.10 Not Recommended in Pediatric Patients Below 3 Years of Age
PREZISTA/ritonavir in pediatric patients below 3 years of age is not recommended in view of toxicity and mortality observed in juvenile rats dosed with darunavir (from 20 mg/kg to 1000 mg/kg) up to days 23 to 26 of age [see Use in Specific Populations (8.1 and 8.4) and Clinical Pharmacology (12.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of labeling:
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Severe Skin Reactions [see Warnings and Precautions (5.3)]
- Diabetes Mellitus/Hyperglycemia [see Warnings and Precautions (5.6)]
- Fat Redistribution [see Warnings and Precautions (5.7)]
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.8)]
- Hemophilia [see Warnings and Precautions (5.9)]
Due to the need for co-administration of PREZISTA with ritonavir, please refer to ritonavir prescribing information for ritonavir-associated adverse reactions.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Treatment Naïve-Adults: TMC114-C211
The safety assessment is based on all safety data from the Phase 3 trial TMC114-C211 comparing PREZISTA/ritonavir 800/100 mg once daily versus lopinavir/ritonavir 800/200 mg per day in 689 antiretroviral treatment-naïve HIV-1-infected adult subjects. The total mean exposure for subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and in the lopinavir/ritonavir 800/200 mg per day arm was 162.5 and 153.5 weeks, respectively.
The majority of the adverse drug reactions (ADRs) reported during treatment with PREZISTA/ritonavir 800/100 mg once daily were mild in severity. The most common clinical ADRs to PREZISTA/ritonavir 800/100 mg once daily (greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, headache, abdominal pain and rash. 2.3% of subjects in the PREZISTA/ritonavir arm discontinued treatment due to ADRs.
ADRs to PREZISTA/ritonavir 800/100 mg once daily of at least moderate intensity (greater than or equal to Grade 2) in antiretroviral treatment-naïve HIV-1-infected adult subjects are presented in Table 6 and subsequent text below the table.
Table 6: Selected Clinical Adverse Drug Reactions to PREZISTA/ritonavir 800/100 mg Once Daily* of at Least Moderate Intensity (≥Grade 2) Occurring in ≥2% of Antiretroviral Treatment-Naïve HIV-1-Infected Adult Subjects (Trial TMC114-C211) System organ class,
preferred term,
%PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC
N=343lopinavir/ritonavir 800/200 mg per day + TDF/FTC
N=346N=total number of subjects per treatment group; FTC=emtricitabine; TDF=tenofovir disoproxil fumarate - * Excluding laboratory abnormalities reported as ADRs.
Gastrointestinal Disorders Abdominal pain 6% 6% Diarrhea 9% 16% Nausea 4% 4% Vomiting 2% 4% General Disorders and Administration Site Conditions Fatigue <1% 3% Metabolism and Nutrition Disorders Anorexia 2% <1% Nervous System Disorders Headache 7% 6% Skin and Subcutaneous Tissue Disorders Rash 6% 7% Less Common Adverse Reactions
Treatment-emergent ADRs of at least moderate intensity (greater than or equal to Grade 2) occurring in less than 2% of antiretroviral treatment-naïve subjects receiving PREZISTA/ritonavir 800/100 mg once daily are listed below by body system:
Gastrointestinal Disorders: acute pancreatitis, dyspepsia, flatulence
General Disorders and Administration Site Conditions: asthenia
Hepatobiliary Disorders: acute hepatitis (e.g., acute hepatitis, cytolytic hepatitis, hepatotoxicity)
Immune System Disorders: (drug) hypersensitivity, immune reconstitution syndrome
Metabolism and Nutrition Disorders: diabetes mellitus
Musculoskeletal and Connective Tissue Disorders: myalgia, osteonecrosis
Psychiatric Disorders: abnormal dreams
Skin and Subcutaneous Tissue Disorders: angioedema, pruritus, Stevens-Johnson Syndrome, urticaria
Laboratory Abnormalities
Selected Grade 2 to 4 laboratory abnormalities that represent a worsening from baseline observed in antiretroviral treatment-naïve adult subjects treated with PREZISTA/ritonavir 800/100 mg once daily are presented in Table 7.
Table 7: Grade 2 to 4 Laboratory Abnormalities Observed in Antiretroviral Treatment-Naïve HIV-1-Infected Adult Subjects* (Trial TMC114-C211) Laboratory parameter
%Limit PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC lopinavir/ritonavir 800/200 mg per day + TDF/FTC N=total number of subjects per treatment group; FTC=emtricitabine; TDF=tenofovir disoproxil fumarate - * Grade 4 data not applicable in Division of AIDS grading scale.
Biochemistry Alanine Aminotransferase Grade 2 >2.5 to ≤5.0 × ULN 9% 9% Grade 3 >5.0 to ≤10.0 × ULN 3% 3% Grade 4 >10.0 × ULN <1% 3% Aspartate Aminotransferase Grade 2 >2.5 to ≤5.0 × ULN 7% 10% Grade 3 >5.0 to ≤10.0 × ULN 4% 2% Grade 4 >10.0 × ULN 1% 3% Alkaline Phosphatase Grade 2 >2.5 to ≤5.0 × ULN 1% 1% Grade 3 >5.0 to ≤10.0 × ULN 0% <1% Grade 4 >10.0 × ULN 0% 0% Hyperbilirubinemia Grade 2 >1.5 to ≤2.5 × ULN <1% 5% Grade 3 >2.5 to ≤5.0 × ULN <1% <1% Grade 4 >5.0 × ULN 0% 0% Triglycerides Grade 2 5.65–8.48 mmol/L
500–750 mg/dL3% 10% Grade 3 8.49–13.56 mmol/L
751–1200 mg/dL2% 5% Grade 4 >13.56 mmol/L
>1200 mg/dL1% 1% Total Cholesterol Grade 2 6.20–7.77 mmol/L
240–300 mg/dL23% 27% Grade 3 >7.77 mmol/L
>300 mg/dL1% 5% Low-Density Lipoprotein Cholesterol Grade 2 4.13–4.90 mmol/L
160–190 mg/dL14% 12% Grade 3 ≥4.91 mmol/L
≥191 mg/dL9% 6% Elevated Glucose Levels Grade 2 6.95–13.88 mmol/L
126–250 mg/dL11% 10% Grade 3 13.89–27.75 mmol/L
251–500 mg/dL1% <1% Grade 4 >27.75 mmol/L
>500 mg/dL0% 0% Pancreatic Lipase Grade 2 >1.5 to ≤3.0 × ULN 3% 2% Grade 3 >3.0 to ≤5.0 × ULN <1% 1% Grade 4 >5.0 × ULN 0% <1% Pancreatic Amylase Grade 2 >1.5 to ≤2.0 × ULN 5% 2% Grade 3 >2.0 to ≤5.0 × ULN 5% 4% Grade 4 >5.0 × ULN 0% <1% Treatment-Experienced Adults: TMC114-C214
The safety assessment is based on all safety data from the Phase 3 trial TMC114-C214 comparing PREZISTA/ritonavir 600/100 mg twice daily versus lopinavir/ritonavir 400/100 mg twice daily in 595 antiretroviral treatment-experienced HIV-1-infected adult subjects. The total mean exposure for subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm and in the lopinavir/ritonavir 400/100 mg twice daily arm was 80.7 and 76.4 weeks, respectively.
The majority of the ADRs reported during treatment with PREZISTA/ritonavir 600/100 mg twice daily were mild in severity. The most common clinical ADRs to PREZISTA/ritonavir 600/100 mg twice daily (greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, nausea, rash, abdominal pain and vomiting. 4.7% of subjects in the PREZISTA/ritonavir arm discontinued treatment due to ADRs.
ADRs to PREZISTA/ritonavir 600/100 mg twice daily of at least moderate intensity (greater than or equal to Grade 2) in antiretroviral treatment-experienced HIV-1-infected adult subjects are presented in Table 8 and subsequent text below the table.
Table 8: Selected Clinical Adverse Drug Reactions to PREZISTA/ritonavir 600/100 mg Twice Daily* of at Least Moderate Intensity (≥Grade 2) Occurring in ≥2% of Antiretroviral Treatment-Experienced HIV-1-Infected Adult Subjects (Trial TMC114-C214) System organ class,
preferred term,
%PREZISTA/ritonavir 600/100 mg twice daily + OBR
N=298lopinavir/ritonavir 400/100 mg twice daily + OBR
N=297N=total number of subjects per treatment group; OBR=optimized background regimen - * Excluding laboratory abnormalities reported as ADRs
Gastrointestinal Disorders Abdominal distension 2% <1% Abdominal pain 6% 3% Diarrhea 14% 20% Dyspepsia 2% 1% Nausea 7% 6% Vomiting 5% 3% General Disorders and Administration Site Conditions Asthenia 3% 1% Fatigue 2% 1% Metabolism and Nutrition Disorders Anorexia 2% 2% Diabetes mellitus 2% <1% Nervous System Disorders Headache 3% 3% Skin and Subcutaneous Tissue Disorders Rash 7% 3% Less Common Adverse Reactions
Treatment-emergent ADRs of at least moderate intensity (greater than or equal to Grade 2) occurring in less than 2% of antiretroviral treatment-experienced subjects receiving PREZISTA/ritonavir 600/100 mg twice daily are listed below by body system:
Gastrointestinal Disorders: acute pancreatitis, flatulence
Musculoskeletal and Connective Tissue Disorders: myalgia
Psychiatric Disorders: abnormal dreams
Skin and Subcutaneous Tissue Disorders: pruritus, urticaria
Laboratory Abnormalities
Selected Grade 2 to 4 laboratory abnormalities that represent a worsening from baseline observed in antiretroviral treatment-experienced adult subjects treated with PREZISTA/ritonavir 600/100 mg twice daily are presented in Table 9.
Table 9: Grade 2 to 4 Laboratory Abnormalities Observed in Antiretroviral Treatment-Experienced HIV-1-Infected Adult Subjects* (Trial TMC114-C214) Laboratory parameter,
%Limit PREZISTA/ritonavir 600/100 mg twice daily + OBR lopinavir/ritonavir 400/100 mg twice daily + OBR N=total number of subjects per treatment group; OBR=optimized background regimen - * Grade 4 data not applicable in Division of AIDS grading scale
Biochemistry Alanine Aminotransferase Grade 2 >2.5 to ≤5.0 × ULN 7% 5% Grade 3 >5.0 to ≤10.0 × ULN 2% 2% Grade 4 >10.0 × ULN 1% 2% Aspartate Aminotransferase Grade 2 >2.5 to ≤5.0 × ULN 6% 6% Grade 3 >5.0 to ≤10.0 × ULN 2% 2% Grade 4 >10.0 × ULN <1% 2% Alkaline Phosphatase Grade 2 >2.5 to ≤5.0 × ULN <1% 0% Grade 3 >5.0 to ≤10.0 × ULN <1% <1% Grade 4 >10.0 × ULN 0% 0% Hyperbilirubinemia Grade 2 >1.5 to ≤2.5 × ULN <1% 2% Grade 3 >2.5 to ≤5.0 × ULN <1% <1% Grade 4 >5.0 × ULN <1% 0% Triglycerides Grade 2 5.65–8.48 mmol/L
500–750 mg/dL10% 11% Grade 3 8.49–13.56 mmol/L
751–1200 mg/dL7% 10% Grade 4 >13.56 mmol/L
>1200 mg/dL3% 6% Total Cholesterol Grade 2 6.20–7.77 mmol/L
240–300 mg/dL25% 23% Grade 3 >7.77 mmol/L
>300 mg/dL10% 14% Low-Density Lipoprotein Cholesterol Grade 2 4.13–4.90 mmol/L
160–190 mg/dL14% 14% Grade 3 ≥4.91 mmol/L
≥191 mg/dL8% 9% Elevated Glucose Levels Grade 2 6.95–13.88 mmol/L
126–250 mg/dL10% 11% Grade 3 13.89–27.75 mmol/L
251–500 mg/dL1% <1% Grade 4 >27.75 mmol/L
>500 mg/dL<1% 0% Pancreatic Lipase Grade 2 >1.5 to ≤3.0 × ULN 3% 4% Grade 3 >3.0 to ≤5.0 × ULN 2% <1% Grade 4 >5.0 × ULN <1% 0% Pancreatic Amylase Grade 2 >1.5 to ≤2.0 × ULN 6% 7% Grade 3 >2.0 to ≤5.0 × ULN 7% 3% Grade 4 >5.0 × ULN 0% 0% Serious ADRs
The following serious ADRs of at least moderate intensity (greater than or equal to Grade 2) occurred in the Phase 2b and Phase 3 trials with PREZISTA/ritonavir: abdominal pain, acute hepatitis, acute pancreatitis, anorexia, asthenia, diabetes mellitus, diarrhea, fatigue, headache, hepatic enzyme increased, hypercholesterolemia, hyperglycemia, hypertriglyceridemia, immune reconstitution syndrome, low density lipoprotein increased, nausea, pancreatic enzyme increased, rash, Stevens-Johnson Syndrome, and vomiting.
Patients Co-Infected with Hepatitis B and/or Hepatitis C Virus
In subjects co-infected with hepatitis B or C virus receiving PREZISTA/ritonavir, the incidence of adverse events and clinical chemistry abnormalities was not higher than in subjects receiving PREZISTA/ritonavir who were not co-infected, except for increased hepatic enzymes [see Warnings and Precautions (5.2)]. The pharmacokinetic exposure in co-infected subjects was comparable to that in subjects without co-infection.
Clinical Trials Experience: Pediatric Patients
PREZISTA/ritonavir has been studied in combination with other antiretroviral agents in 3 Phase 2 trials. TMC114-C212, in which 80 antiretroviral treatment-experienced HIV-1-infected pediatric subjects 6 to less than 18 years of age and weighing at least 20 kg were included, TMC114-C228, in which 21 antiretroviral treatment-experienced HIV-1-infected pediatric subjects 3 to less than 6 years of age and weighing at least 10 kg were included, and TMC114-C230 in which 12 antiretroviral treatment-naïve HIV-1 infected pediatric patients aged from 12 to less than 18 years and weighing at least 40 kg were included. The TMC114-C212 and C228 trials evaluated PREZISTA/ritonavir twice daily dosing and the TMC114-C230 trial evaluated PREZISTA/ritonavir once daily dosing [see Use in Specific Populations (8.4) and Clinical Studies (14.4)].
Frequency, type, and severity of ADRs in pediatric subjects were comparable to those observed in adults.
TMC114-C212
Clinical ADRs to PREZISTA/ritonavir (all grades, greater than or equal to 3%), were vomiting (13%), diarrhea (11%), abdominal pain (10%), headache (9%), rash (5%), nausea (4%), and fatigue (3%).
Grade 3 or 4 laboratory abnormalities were ALT increased (Grade 3: 3%; Grade 4: 1%), AST increased (Grade 3: 1%), pancreatic amylase increased (Grade 3: 4%, Grade 4: 1%), pancreatic lipase increased (Grade 3: 1%), total cholesterol increased (Grade 3: 1%), and LDL increased (Grade 3: 3%).
TMC114-C228
Clinical ADRs to PREZISTA/ritonavir (all grades, greater than or equal to 5%), were diarrhea (24%), vomiting (19%), rash (19%), abdominal pain (5%), and anorexia (5%).
There were no Grade 3 or 4 laboratory abnormalities considered as ADRs in this trial.
TMC114-C230
Clinical ADRs to PREZISTA/ritonavir (all grades, greater than or equal to 3%), were vomiting (33%), nausea (25%), diarrhea (16.7%), abdominal pain (8.3%), decreased appetite (8.3%), pruritus (8.3%), and rash (8.3%).
There were no Grade 3 or 4 laboratory abnormalities considered as ADRs in this trial.
6.2 Postmarketing Experience
The following events have been identified during post approval use of PREZISTA. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Redistribution of body fat has been reported.
Rarely, rhabdomyolysis (associated with co-administration with HMG-CoA reductase inhibitors and PREZISTA/ritonavir) has been reported.
In addition, toxic epidermal necrolysis, acute generalized exanthematous pustulosis and drug rash with eosinophilia and systemic symptoms have been reported rarely [see Warnings and Precautions (5.3)].
-
7 DRUG INTERACTIONS
7.1 Potential for PREZISTA/ritonavir to Affect Other Drugs
PREZISTA co-administered with ritonavir is an inhibitor of CYP3A, CYP2D6, and P-gp. Co-administration of PREZISTA and ritonavir with drugs that are primarily metabolized by CYP3A and CYP2D6 or are transported by P-gp may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and adverse events (see Table 10).
7.2 Potential for Other Drugs to Affect Darunavir
Darunavir and ritonavir are metabolized by CYP3A. In vitro data indicate that darunavir may be a P-gp substrate. Drugs that induce CYP3A activity would be expected to increase the clearance of darunavir and ritonavir, resulting in lowered plasma concentrations of darunavir and ritonavir. Co-administration of darunavir and ritonavir and other drugs that inhibit CYP3A, or P-gp may decrease the clearance of darunavir and ritonavir and may result in increased plasma concentrations of darunavir and ritonavir (see Table 10).
7.3 Established and Other Potentially Significant Drug Interactions
Table 10 provides dosing recommendations as a result of drug interactions with PREZISTA/ritonavir. These recommendations are based on either drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of efficacy. The table includes potentially significant interactions but is not all inclusive [see Contraindications (4) and Clinical Pharmacology (12.3)].
Table 10: Established and Other Potentially Significant Drug Interactions: Alterations in Dose or Regimen May be Recommended Based on Drug Interaction Studies or Predicted Interaction (see Contraindications (4) for a complete list of contraindicated drugs) [see Clinical Pharmacology (12.3) for Magnitude of Interaction, Tables 15 and 16] Concomitant Drug Class
Drug NameEffect on Concentration of Darunavir Or Concomitant Drug Clinical Comment HIV-1-Antiviral Agents: Nucleoside Reverse Transcriptase Inhibitors (NRTIs) didanosine ↔ darunavir
↔ didanosineDidanosine should be administered one hour before or two hours after PREZISTA/ritonavir (which are administered with food). HIV-1-Antiviral Agents: HIV-Protease Inhibitors (PIs) indinavir ↑ darunavir
↑ indinavirThe appropriate dose of indinavir in combination with PREZISTA/ritonavir has not been established. (The reference regimen for indinavir was indinavir/ritonavir 800/100 mg twice daily.) lopinavir/ritonavir ↓ darunavir
↔ lopinavirAppropriate doses of the combination have not been established. Hence, it is not recommended to co-administer lopinavir/ritonavir and PREZISTA, with or without ritonavir. saquinavir ↓ darunavir
↔ saquinavirAppropriate doses of the combination have not been established. Hence, it is not recommended to co-administer saquinavir and PREZISTA, with or without ritonavir. Other HIV protease inhibitors, except atazanavir [see Drug Interactions (7.4)] As co-administration with PREZISTA/ritonavir has not been studied, co-administration is not recommended. HIV-1-Antiviral Agents: CCR5 co-receptor antagonists maraviroc ↑ maraviroc When used in combination with PREZISTA/ritonavir, the dose of maraviroc should be 150 mg twice daily. Other Agents Alpha 1-adrenoreceptor antagonist: alfuzosin ↑ alfuzosin Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as hypotension. Antibacterial:
clarithromycin↔ darunavir
↑ clarithromycinNo dose adjustment of the combination is required for patients with normal renal function. For co-administration of clarithromycin and PREZISTA/ritonavir in patients with renal impairment, the following dose adjustments should be considered: - For subjects with CLcr of 30–60 mL/min, the dose of clarithromycin should be reduced by 50%.
- For subjects with CLcr of <30 mL/min, the dose of clarithromycin should be reduced by 75%.
Anticoagulants:
Direct Oral Anticoagulants (DOACs)apixaban ↑ apixaban Due to potentially increased bleeding risk, dosing recommendations for co-administration of apixaban with PREZISTA depends on the apixaban dose. Refer to apixaban dosing instructions for co-administration with strong CYP3A and P-gp inhibitors in apixaban prescribing information. rivaroxaban ↑ rivaroxaban Co-administration of PREZISTA/ritonavir and rivaroxaban is not recommended because it may lead to an increased bleeding risk. betrixaban
dabigatran
edoxaban↔ betrixaban
↔ dabigatran
↔ edoxabanNo dose adjustment is needed when betrixaban, dabigatran, or edoxaban is co-administered with PREZISTA. Other Anticoagulants warfarin ↓ warfarin
↔ darunavirWarfarin concentrations are decreased when co-administered with PREZISTA/ritonavir. It is recommended that the international normalized ratio (INR) be monitored when warfarin is combined with PREZISTA/ritonavir. Anticonvulsants:
carbamazepine↔ darunavir
↑ carbamazepineThe dose of either PREZISTA/ritonavir or carbamazepine does not need to be adjusted when initiating co-administration with PREZISTA/ritonavir and carbamazepine. Clinical monitoring of carbamazepine concentrations and its dose titration is recommended to achieve the desired clinical response. clonazepam ↑ clonazepam Clinical monitoring of anticonvulsants that are metabolized by CYP3A is recommended. phenobarbital, phenytoin ↔ darunavir
↓ phenytoin
↓ phenobarbitalPhenytoin and phenobarbital levels should be monitored when co-administering with PREZISTA/ritonavir. Antidepressants:
Selective Serotonin Reuptake Inhibitors (SSRIs):
paroxetine, sertraline↓ paroxetine
↓ sertralineIf either sertraline or paroxetine is initiated in patients receiving PREZISTA/ritonavir, dose titrating the SSRI based on a clinical assessment of antidepressant response is recommended. Monitor for antidepressant response in patients on a stable dose of sertraline or paroxetine who start treatment with PREZISTA/ritonavir. Tricyclic Antidepressants (TCAs): amitriptyline, desipramine, imipramine, nortriptyline ↑ amitriptyline
↑ desipramine
↑ imipramine
↑ nortriptylineUse a lower dose of the tricyclic antidepressants and trazodone due to potential increased adverse events such as nausea, dizziness, hypotension and syncope. Other: trazodone ↑ trazodone Antifungals:
itraconazole, isavuconazole, ketoconazole, posaconazole↑ darunavir
↑ itraconazole
↑ isavuconazole
↑ ketoconazole
↔ posaconazoleMonitor for increased PREZISTA/ritonavir and/or antifungal adverse events with concomitant use of these antifungals. When co-administration is required, the daily dose of ketoconazole or itraconazole should not exceed 200 mg with monitoring for increased antifungal adverse events. voriconazole ↓ voriconazole Voriconazole is not recommended for patients receiving PREZISTA/ritonavir unless an assessment comparing predicted benefit to risk ratio justifies the use of voriconazole. Anti-gout:
colchicine↑ colchicine Co-administration is contraindicated in patients with renal and/or hepatic impairment due to potential for serious and/or life-threatening reactions. For patients without renal or hepatic impairment:
-
Treatment of gout-flares – co-administration of colchicine in patients on PREZISTA/ritonavir:
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Treatment course to be repeated no earlier than 3 days. -
Prophylaxis of gout-flares – co-administration of colchicine in patients on PREZISTA/ritonavir:
If the original regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day.
If the original regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. -
Treatment of familial Mediterranean fever – co-administration of colchicine in patients on PREZISTA/ritonavir:
maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day).
Antimalarial:
artemether/lumefantrine↓ artemether
↓ dihydroartemisinin
↑ lumefantrine
↔ darunavirThe combination of PREZISTA/ritonavir and artemether/lumefantrine can be used without dose adjustments. However, the combination should be used with caution as increased lumefantrine exposure may increase the risk of QT prolongation. Antimycobacterials: rifampin ↓ darunavir Co-administration is contraindicated due to potential for loss of therapeutic effect and development of resistance. rifabutin
(The reference regimen for rifabutin was 300 mg once daily.)↑ darunavir
↑ rifabutin
↑ 25-O-desacetylrifabutinDose reduction of rifabutin by at least 75% of the usual dose (300 mg once daily) is recommended (i.e., a maximum dose of 150 mg every other day). Increased monitoring for adverse events is warranted in patients receiving this combination and further dose reduction of rifabutin may be necessary. rifapentine ↓ darunavir Co-administration of PREZISTA/ritonavir with rifapentine is not recommended. Antineoplastics:
dasatinib, nilotinib↑ antineoplastics A decrease in the dosage or an adjustment of the dosing interval of dasatinib and nilotinib may be necessary for patients. Please refer to the dasatinib and nilotinib prescribing information for dosing instructions. vinblastine, vincristine For vincristine and vinblastine, consideration should be given to temporarily withholding the ritonavir-containing antiretroviral regimen in patients who develop significant hematologic or gastrointestinal side effects when PREZISTA/ritonavir is administered concurrently with vincristine or vinblastine. If the antiretroviral regimen must be withheld for a prolonged period, consideration should be given to initiating a revised regimen that does not include a CYP3A or P-gp inhibitor. Antipsychotics: lurasidone ↑ lurasidone Co-administration is contraindicated due to potential for serious and/or life-threatening reactions. pimozide ↑ pimozide Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. quetiapine ↑ quetiapine Initiation of PREZISTA with ritonavir in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in quetiapine exposures. If co-administration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring.
Initiation of quetiapine in patients taking PREZISTA with ritonavir:
Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine.e.g. perphenazine, risperidone, thioridazine ↑ antipsychotics A decrease in the dose of antipsychotics that are metabolized by CYP3A or CYP2D6 may be needed when co-administered with PREZISTA/ritonavir. β-Blockers:
e.g. carvedilol, metoprolol, timolol↑ beta-blockers Clinical monitoring of patients is recommended. A dose decrease may be needed for these drugs when co-administered with PREZISTA/ritonavir and a lower dose of the beta blocker should be considered. Calcium Channel Blockers:
amlodipine, diltiazem, felodipine, nicardipine, nifedipine, verapamil↑ calcium channel blockers Clinical monitoring of patients is recommended. Cardiac Disorders: ranolazine, ivabradine ↑ ranolazine
↑ ivabradineCo-administration is contraindicated due to potential for serious and/or life-threatening reactions. dronedarone ↑ dronedarone Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. Other antiarrhythmics
e.g. amiodarone, bepridil, disopyramide, flecainide, lidocaine (systemic), mexiletine, propafenone, quinidine↑ antiarrhythmics Therapeutic concentration monitoring, if available, is recommended for antiarrhythmics when co-administered with PREZISTA/ritonavir. digoxin ↑ digoxin The lowest dose of digoxin should initially be prescribed. The serum digoxin concentrations should be monitored and used for titration of digoxin dose to obtain the desired clinical effect. Systemic/Inhaled/ Nasal/Ophthalmic Corticosteroids:
e.g.
betamethasone
budesonide
ciclesonide
dexamethasone
fluticasone
methylprednisolone
mometasone
triamcinolone↓ darunavir
↑ corticosteroidsCo-administration of PREZISTA/ritonavir with systemic dexamethasone or other systemic corticosteroids that induce CYP3A may result in loss of therapeutic effect and development of resistance to darunavir. Consider alternative corticosteroids. Co-administration with corticosteroids of which exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing's syndrome and adrenal suppression.
Alternative corticosteroids including beclomethasone, prednisone, and prednisolone (for which PK and/or PD are less affected by strong CYP3A inhibitors relative to other steroids) should be considered, particularly for long term use.Endothelin receptor antagonist: bosentan ↑ bosentan Co-administration of bosentan in patients on PREZISTA/ritonavir:
In patients who have been receiving PREZISTA/ritonavir for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability.Co-administration of PREZISTA/ritonavir in patients on bosentan:
Discontinue use of bosentan at least 36 hours prior to initiation of PREZISTA/ritonavir. After at least 10 days following the initiation of PREZISTA/ritonavir, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability.Ergot derivatives: e.g. dihydroergotamine, ergotamine, methylergonovine ↑ ergot derivatives Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. GI motility agent: cisapride ↑ cisapride Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. Hepatitis C virus (HCV): Direct-Acting Antivirals:
elbasvir/grazoprevir↑ elbasvir/grazoprevir Co-administration is contraindicated due to potential for the increased risk of alanine transaminase (ALT) elevations. glecaprevir/pibrentasvir ↑ glecaprevir
↑ pibrentasvirCo-administration of PREZISTA/ritonavir with glecaprevir/pibrentasvir is not recommended. simeprevir ↑ simeprevir
↑ darunavirCo-administration of PREZISTA/ritonavir and simeprevir is not recommended Herbal product: St. John's wort (Hypericum perforatum) ↓ darunavir Co-administration is contraindicated due to potential for reduced plasma concentrations of darunavir, which may result in loss of therapeutic effect and development of resistance. Hormonal contraceptives: Effective alternative (non-hormonal) contraceptive method or a barrier method of contraception is recommended [see Use in Specific Populations (8.3)]. ethinyl estradiol, norethindrone, drospirenone ↓ ethinyl estradiol
↓ norethindrone
drospirenone: effects unknownFor co-administration with drospirenone, clinical monitoring is recommended due to the potential for hyperkalemia.
No data are available to make recommendations on co-administration with other hormonal contraceptives.Immunosuppressants:
e.g. cyclosporine, tacrolimus, sirolimus↑ immunosuppressants Therapeutic concentration monitoring of the immunosuppressive agent is recommended when co-administered with PREZISTA/ritonavir. Immunosuppressant/neoplastic:
everolimusCo-administration of everolimus and PREZISTA/ritonavir is not recommended. irinotecan Discontinue PREZISTA/ritonavir at least 1 week prior to starting irinotecan therapy. Do not administer PREZISTA/ritonavir with irinotecan unless there are no therapeutic alternatives. Inhaled beta agonist: salmeterol ↑ salmeterol Co-administration of salmeterol and PREZISTA/ritonavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. Lipid Modifying Agents: HMG-CoA reductase inhibitors: lovastatin, simvastatin ↑ lovastatin
↑ simvastatinCo-administration is contraindicated due to potential for serious reactions such as myopathy including rhabdomyolysis. atorvastatin, pravastatin, rosuvastatin ↑ HMG-CoA reductase inhibitors Co-administration of PREZISTA/ritonavir with HMG-Co A reductase inhibitors may lead to adverse events such as myopathy. Titrate atorvastatin, pravastatin or rosuvastatin dose carefully and use the lowest necessary dose while monitoring for adverse events. Do not exceed atorvastatin 20 mg/day. Other lipid modifying agents: lomitapide ↑ lomitapide Co-administration is contraindicated due to potential for markedly increased transaminases. Narcotic analgesics metabolized by CYP3A: e.g. fentanyl, oxycodone ↑ fentanyl
↑ oxycodoneCareful monitoring of therapeutic effects and adverse reactions associated with CYP3A-metabolized narcotic analgesics (including potentially fatal respiratory depression) is recommended with co-administration. tramadol ↑ tramadol A dose decrease may be needed for tramadol with concomitant use. Narcotic analgesics/treatment of opioid dependence:
buprenorphine, buprenorphine/naloxone↔ buprenorphine, naloxone
↑ norbuprenorphine (metabolite)No dose adjustment for buprenorphine or buprenorphine/naloxone is required with concurrent administration of PREZISTA/ritonavir. Clinical monitoring is recommended if PREZISTA/ritonavir and buprenorphine or buprenorphine/naloxone are co-administered. methadone ↓ methadone No adjustment of methadone dosage is required when initiating co-administration of PREZISTA/ritonavir. However, clinical monitoring is recommended as the dose of methadone during maintenance therapy may need to be adjusted in some patients. Opioid Antagonist naloxegol ↑ naloxegol Co-administration of PREZISTA/ritonavir and naloxegol is contraindicated due to potential for precipitating opioid withdrawal symptoms. PDE-5 inhibitors:
e.g. avanafil, sildenafil, tadalafil, vardenafil↑ PDE-5 inhibitors (only the use of sildenafil at doses used for treatment of erectile dysfunction has been studied with PREZISTA/ritonavir) Co-administration with PREZISTA/ritonavir may result in an increase in PDE-5 inhibitor-associated adverse events, including hypotension, syncope, visual disturbances and priapism.
Use of PDE-5 inhibitors for pulmonary arterial hypertension (PAH):
Co-administration with sildenafil used for PAH is contraindicated due to potential for sildenafil associated adverse reactions (which include visual disturbances, hypotension, prolonged erection, and syncope).
The following dose adjustments are recommended for use of tadalafil with PREZISTA/ritonavir:-
Co-administration of tadalafil in patients on PREZISTA/ritonavir:
In patients receiving PREZISTA/ritonavir for at least one week, start tadalafil at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability. -
Co-administration of PREZISTA/ritonavir in patients on tadalafil:
Avoid use of tadalafil during the initiation of PREZISTA/ritonavir. Stop tadalafil at least 24 hours prior to starting PREZISTA/ritonavir. After at least one week following the initiation of PREZISTA/ritonavir, resume tadalafil at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.
Use of PDE-5 inhibitors for erectile dysfunction:
Sildenafil at a single dose not exceeding 25 mg in 48 hours, vardenafil at a single dose not exceeding 2.5 mg dose in 72 hours, or tadalafil at a single dose not exceeding 10 mg dose in 72 hours can be used with increased monitoring for PDE-5 inhibitor-associated adverse events.Co-administration of PREZISTA/ritonavir and avanafil is not recommended.
Platelet aggregation inhibitor:
ticagrelor↑ ticagrelor Co-administration of PREZISTA/ritonavir and ticagrelor is not recommended. Proton pump inhibitor:
omeprazole↓ omeprazole
↔ darunavirWhen omeprazole is co-administered with PREZISTA/ritonavir, monitor patients for decreased efficacy of omeprazole. Consider increasing the omeprazole dose in patients whose symptoms are not well controlled; avoid use of more than 40 mg per day of omeprazole. Sedatives/hypnotics: orally administered midazolam, triazolam ↑ midazolam
↑ triazolamCo-administration is contraindicated due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. Triazolam and orally administered midazolam are extensively metabolized by CYP3A. Co-administration of triazolam or orally administered midazolam with PREZISTA may cause large increases in the concentrations of these benzodiazepines. metabolized by CYP3A
e.g. buspirone, diazepam, estazolam, zolpidem↑ sedatives/hypnotics Titration is recommended when co-administering PREZISTA/ritonavir with sedatives/hypnotics metabolized by CYP3A and a lower dose of the sedatives/hypnotics should be considered with monitoring for adverse events. parenterally administered midazolam Co-administration of parenteral midazolam should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered. Urinary antispasmodics fesoterodine ↑ fesoterodine When fesoterodine is co-administered with PREZISTA/ritonavir, do not exceed a fesoterodine dose of 4 mg once daily. solifenacin ↑ solifenacin When solifenacin is co-administered with PREZISTA/ritonavir, do not exceed a solifenacin dose of 5 mg once daily. 7.4 Drugs without Clinically Significant Interactions with PREZISTA
No dosage adjustments are recommended when PREZISTA/ritonavir is co-administered with the following medications: atazanavir, dolutegravir, efavirenz, etravirine, nevirapine, nucleoside reverse transcriptase inhibitors (abacavir, emtricitabine, emtricitabine/tenofovir alafenamide, lamivudine, stavudine, tenofovir disoproxil fumarate, zidovudine), pitavastatin, raltegravir, ranitidine, or rilpivirine.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to PREZISTA during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) 1-800-258-4263.
Risk Summary
Available limited data from the APR show no difference in rate of overall birth defects for darunavir (2.7%) compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) [see Data]. The APR uses the MACDP as the U.S. reference population for birth defects in the general population. The MACDP evaluates women and infants from a limited geographic area and does not include outcomes for births that occurred at less than 20 weeks gestation.
The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15-20%. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Studies in animals did not show evidence of developmental toxicity. Exposures (based on AUC) in rats were 3-fold higher, whereas in mice and rabbits, exposures were lower (less than 1-fold) than human exposures at the recommended daily dose [see Data].
Clinical Considerations
The recommended dosage in pregnant patients is PREZISTA 600 mg taken with ritonavir 100 mg twice daily with food.
PREZISTA 800 mg taken with ritonavir 100 mg once daily should only be considered in certain pregnant patients who are already on a stable PREZISTA 800 mg with ritonavir 100 mg once daily regimen prior to pregnancy, are virologically suppressed (HIV-1 RNA less than 50 copies per mL), and in whom a change to twice daily PREZISTA 600 mg with ritonavir 100 mg may compromise tolerability or compliance [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Data
Human Data
PREZISTA/ritonavir (600/100 mg twice daily or 800/100 mg once daily) in combination with a background regimen was evaluated in a clinical trial of 36 pregnant women during the second and third trimesters, and postpartum. Eighteen subjects were enrolled in each BID and QD treatment arms. Twenty-nine subjects completed the trial through the postpartum period (6–12 weeks after delivery) and 7 subjects discontinued before trial completion, 5 subjects in the BID arm and 2 subjects in the QD arm.
The pharmacokinetic data demonstrate that exposure to darunavir and ritonavir as part of an antiretroviral regimen was lower during pregnancy compared with postpartum (6–12 weeks). Exposure reductions during pregnancy were greater for the once daily regimen as compared to the twice daily regimen [see Clinical Pharmacology (12.3)].
Virologic response was preserved. In the BID arm, the proportion of subjects with HIV-1 RNA <50 copies/mL were 39% (7/18) at baseline, 61% (11/18) through the third trimester visit, and 61% (11/18) through the 6–12 week postpartum visit. Virologic outcomes during the third trimester visit showed HIV-1 RNA ≥50 copies/mL for 11% (2/18) of subjects and were missing for 5 subjects (1 subject discontinued prematurely due to virologic failure). In the QD arm, the proportion of subjects with HIV-1 RNA <50 copies/mL were 61% (11/18) at baseline, 83% (15/18) through the third trimester visit, and 78% (14/18) through the 6–12 week postpartum visit. Virologic outcomes during the third trimester visit showed HIV-1 RNA ≥50 copies/mL for none of the subjects and were missing for 3 subjects (1 subject discontinued prematurely due to virologic failure).
PREZISTA/ritonavir was well tolerated during pregnancy and postpartum. There were no new clinically relevant safety findings compared with the known safety profile of PREZISTA/ritonavir in HIV-1-infected adults.
Among the 31 infants with HIV test results available data, born to the 31 HIV-infected pregnant women who completed trial through delivery or postpartum period, all 31 infants had test results that were negative for HIV-1 at the time of delivery and/or through 16 weeks postpartum. All 31 infants received antiretroviral prophylactic treatment containing zidovudine.
Based on prospective reports to the APR of 615 live births following exposure to darunavir-containing regimens during pregnancy (including 385 exposed in the first trimester and 230 exposed in the second/third trimester), there was no difference in rate of overall birth defects for darunavir compared with the background rate for major birth defects in a U.S. reference population of the MACDP.
The prevalence of birth defects in live births was 2.6% (95% CI: 1.2% to 4.7%) with first trimester exposure to darunavir containing regimens and 1.7% (95% CI: 0.5% to 4.4%) with second/third trimester exposure to darunavir containing regimens.
Animal Data
Reproduction studies conducted with darunavir showed no embryotoxicity or teratogenicity in mice (doses up to 1000 mg/kg from gestation day (GD) 6-15 with darunavir alone) and rats (doses up to 1000 mg/kg from GD 7-19 in the presence or absence of ritonavir) as well as in rabbits (doses up to 1000 mg/kg/day from GD 8-20 with darunavir alone). In these studies, darunavir exposures (based on AUC) were higher in rats (3-fold), whereas in mice and rabbits, exposures were lower (less than 1-fold) compared to those obtained in humans at the recommended clinical dose of darunavir boosted with ritonavir.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV.
There are no data on the presence of darunavir in human milk, the effects on the breastfed infant, or the effects on milk production. Darunavir is present in the milk of lactating rats [see Data]. Because of the potential for (1) HIV transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants) and (3) serious adverse reactions in a breastfed infant, instruct mothers not to breastfeed if they are receiving PREZISTA [see Use in Specific Populations (8.4)].
Data
Animal Data
Studies in rats (with darunavir alone or with ritonavir) have demonstrated that darunavir is secreted in the milk. In the rat pre- and postnatal development study, a reduction in pup body weight gain was observed due to exposure of pups to drug substances via milk. The maximal maternal plasma exposures achieved with darunavir (up to 1000 mg/kg with ritonavir) were approximately 50% of those obtained in humans at the recommended clinical dose with ritonavir.
8.3 Females and Males of Reproductive Potential
Contraception
Use of PREZISTA may reduce the efficacy of combined hormonal contraceptives and the progestin only pill. Advise patients to use an effective alternative (non-hormonal) contraceptive method or add a barrier method of contraception. For co-administration with drospirenone, clinical monitoring is recommended due to the potential for hyperkalemia [see Drug Interactions (7.3)].
8.4 Pediatric Use
PREZISTA/ritonavir is not recommended in pediatric patients below 3 years of age because of toxicity and mortality observed in juvenile rats dosed with darunavir (from 20 mg/kg to 1000 mg/kg) up to days 23 to 26 of age [see Warnings and Precautions (5.10), Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
The safety, pharmacokinetic profile, and virologic and immunologic responses of PREZISTA/ritonavir administered twice daily were evaluated in treatment-experienced HIV-1-infected pediatric subjects 3 to less than 18 years of age and weighting at least 10 kg. These subjects were evaluated in clinical trials TMC114-C212 (80 subjects, 6 to less than 18 years of age) and TMC114-228 (21 subjects, 3 to less than 6 years of age) [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14.4)]. Frequency, type, and severity of adverse drug reactions in pediatric subjects were comparable to those observed in adults [see Adverse Reactions (6.1)]. Refer to Dosage and Administration (2.5) for twice-daily dosing recommendations for pediatric subjects 3 to less than 18 years of age and weighing at least 10 kg.
In clinical trial TMC114-C230, the safety, pharmacokinetic profile and virologic and immunologic responses of PREZISTA/ritonavir administered once daily were evaluated in treatment-naïve HIV-1 infected pediatric subjects 12 to less than 18 years of age (12 subjects) [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14.4)]. Frequency, type, and severity of adverse drug reactions in pediatric subjects were comparable to those observed in adults [see Adverse Reactions (6.1)]. Once daily dosing recommendations for pediatric patients 3 to less than 12 years of age were derived using population pharmacokinetic modeling and simulation. Although a PREZISTA/ritonavir once daily dosing pediatric trial was not conducted in children less than 12 years of age, there is sufficient clinical safety data to support the predicted PREZISTA exposures for the dosing recommendations in this age group [see Clinical Pharmacology (12.3)]. Please see Dosage and Administration (2.5) for once-daily dosing recommendations for pediatric subjects 3 to less than 18 years of age and weighing at least 10 kg.
Juvenile Animal Data
In a juvenile toxicity study where rats were directly dosed with darunavir (up to 1000 mg/kg), deaths occurred from post-natal day 5 at plasma exposure levels ranging from 0.1 to 1.0 of the human exposure levels. In a 4-week rat toxicology study, when dosing was initiated on post-natal day 23 (the human equivalent of 2 to 3 years of age), no deaths were observed with a plasma exposure (in combination with ritonavir) 2 times the human plasma exposure levels.
8.5 Geriatric Use
Clinical studies of PREZISTA did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. In general, caution should be exercised in the administration and monitoring of PREZISTA in elderly patients, reflecting the greater frequency of decreased hepatic function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
No dosage adjustment of PREZISTA/ritonavir is necessary for patients with either mild or moderate hepatic impairment. No pharmacokinetic or safety data are available regarding the use of PREZISTA/ritonavir in subjects with severe hepatic impairment. Therefore, PREZISTA/ritonavir is not recommended for use in patients with severe hepatic impairment [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV-infected subjects with moderate renal impairment (CrCL between 30–60 mL/min, n=20). No pharmacokinetic data are available in HIV-1-infected patients with severe renal impairment or end stage renal disease; however, because the renal clearance of darunavir is limited, a decrease in total body clearance is not expected in patients with renal impairment. As darunavir and ritonavir are highly bound to plasma proteins, it is unlikely that they will be significantly removed by hemodialysis or peritoneal dialysis [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Human experience of acute overdose with PREZISTA/ritonavir is limited. No specific antidote is available for overdose with PREZISTA. Treatment of overdose with PREZISTA consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. Since PREZISTA is highly protein bound, dialysis is unlikely to be beneficial in significant removal of the active substance.
-
11 DESCRIPTION
PREZISTA (darunavir) is an inhibitor of the human immunodeficiency virus (HIV-1) protease.
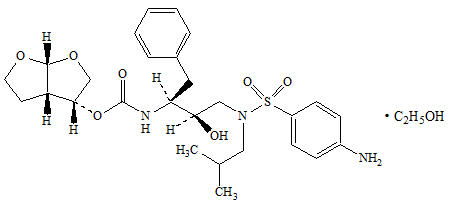
PREZISTA (darunavir), in the form of darunavir ethanolate, has the following chemical name: [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-carbamic acid (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester monoethanolate. Its molecular formula is C27H37N3O7S ∙ C2H5OH and its molecular weight is 593.73. Darunavir ethanolate has the following structural formula:
Darunavir ethanolate is a white to off-white powder with a solubility of approximately 0.15 mg per mL in water at 20°C.
PREZISTA 100 mg per mL oral suspension is available as a white to off-white opaque suspension for oral administration.
Each mL of the oral suspension contains darunavir ethanolate equivalent to 100 mg darunavir. In addition, each mL contains the inactive ingredients citric acid monohydrate, hydrochloric acid (for pH adjustment), hydroxypropyl cellulose, masking flavor, methylparaben sodium, microcrystalline cellulose, purified water, sodium carboxymethylcellulose, strawberry cream flavor and sucralose.
PREZISTA 75 mg tablets are available as white, caplet-shaped, film-coated tablets for oral administration. Each 75 mg tablet contains darunavir ethanolate equivalent to 75 mg of darunavir.
PREZISTA 150 mg tablets are available as white, oval-shaped, film-coated tablets for oral administration. Each 150 mg tablet contains darunavir ethanolate equivalent to 150 mg of darunavir.
PREZISTA 600 mg tablets are available as orange, oval-shaped, film-coated tablets for oral administration. Each 600 mg tablet contains darunavir ethanolate equivalent to 600 mg of darunavir.
PREZISTA 800 mg tablets are available as dark red, oval-shaped, film-coated tablets for oral administration. Each 800 mg tablet contains darunavir ethanolate equivalent to 800 mg of darunavir.
During storage, partial conversion from ethanolate to hydrate may occur; however, this does not affect product quality or performance. Each tablet also contains the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and microcrystalline cellulose. The 800 mg tablet also contains hypromellose. The 75 and 150 mg tablet film coating, OPADRY® White, contains polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide. The 600 mg tablet film coating, OPADRY® Orange, contains FD&C Yellow No. 6, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide. The 800 mg tablet film coating, OPADRY® Dark Red, contains iron oxide red, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide.
All dosages for PREZISTA are expressed in terms of the free form of darunavir.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Pharmacokinetics in Adults
General
Darunavir is primarily metabolized by CYP3A. Ritonavir inhibits CYP3A, thereby increasing the plasma concentrations of darunavir. When a single dose of PREZISTA 600 mg was given orally in combination with 100 mg ritonavir twice daily, there was an approximate 14-fold increase in the systemic exposure of darunavir. Therefore, PREZISTA should only be used in combination with 100 mg of ritonavir to achieve sufficient exposures of darunavir.
The pharmacokinetics of darunavir, co-administered with low dose ritonavir (100 mg), has been evaluated in healthy adult volunteers and in HIV-1-infected subjects. Table 11 displays the population pharmacokinetic estimates of darunavir after oral administration of PREZISTA/ritonavir 600/100 mg twice daily (based on sparse sampling in 285 patients in trial TMC114-C214, 278 patients in trial TMC114-C229 and 119 patients [integrated data] from trials TMC114-C202 and TMC114-C213) and PREZISTA/ritonavir 800/100 mg once daily (based on sparse sampling in 335 patients in trial TMC114-C211 and 280 patients in trial TMC114-C229) to HIV-1-infected patients.
Table 11: Population Pharmacokinetic Estimates of Darunavir at PREZISTA/ritonavir 800/100 mg Once Daily (Trial TMC114-C211, 48-Week Analysis and Trial TMC114-C229, 48-Week Analysis) and PREZISTA/ritonavir 600/100 mg Twice Daily (Trial TMC114-C214, 48-Week Analysis, Trial TMC114-C229, 48-Week Analysis and Integrated Data from Trials TMC114-C213 and TMC114-C202, Primary 24-Week Analysis) PREZISTA / ritonavir
800/100 mg once dailyPREZISTA / ritonavir
600/100 mg twice dailyParameter TMC114-C211
N=335TMC114-C229
N=280TMC114-C214
N=285TMC114-C229
N=278TMC114-C213 + TMC114-C202 (integrated data)
N=119N=number of subjects with data - * AUC24h is calculated as AUC12h*2.
AUC24h (ng.h/mL)* Mean ± Standard Deviation 93026 ± 27050 93334 ± 28626 116796 ± 33594 114302 ± 32681 124698 ± 32286 Median
(Range)87854
(45000–219240)87788
(45456–236920)111632
(64874–355360)109401
(48934–323820)123336
(67714–212980)C0h (ng/mL) Mean ± Standard Deviation 2282 ± 1168 2160 ± 1201 3490 ± 1401 3386 ± 1372 3578 ± 1151 Median
(Range)2041
(368–7242)1896
(184–7881)3307
(1517–13198)3197
(250–11865)3539
(1255–7368)Absorption and Bioavailability
Darunavir, co-administered with 100 mg ritonavir twice daily, was absorbed following oral administration with a Tmax of approximately 2.5–4 hours. The absolute oral bioavailability of a single 600 mg dose of darunavir alone and after co-administration with 100 mg ritonavir twice daily was 37% and 82%, respectively. In vivo data suggest that PREZISTA/ritonavir is an inhibitor of the P-glycoprotein (P-gp) transporters.
Effects of Food on Oral Absorption
When PREZISTA tablets were administered with food, the Cmax and AUC of darunavir, co-administered with ritonavir, is approximately 40% higher relative to the fasting state. Within the range of meals studied, darunavir exposure is similar. The total caloric content of the various meals evaluated ranged from 240 Kcal (12 gms fat) to 928 Kcal (56 gms fat).
Distribution
Darunavir is approximately 95% bound to plasma proteins. Darunavir binds primarily to plasma alpha 1-acid glycoprotein (AAG).
Metabolism
In vitro experiments with human liver microsomes (HLMs) indicate that darunavir primarily undergoes oxidative metabolism. Darunavir is extensively metabolized by CYP enzymes, primarily by CYP3A. A mass balance study in healthy volunteers showed that after a single dose administration of 400 mg 14C-darunavir, co-administered with 100 mg ritonavir, the majority of the radioactivity in the plasma was due to darunavir. At least 3 oxidative metabolites of darunavir have been identified in humans; all showed activity that was at least 90% less than the activity of darunavir against wild-type HIV-1.
Elimination
A mass balance study in healthy volunteers showed that after single dose administration of 400 mg 14C-darunavir, co-administered with 100 mg ritonavir, approximately 79.5% and 13.9% of the administered dose of 14C-darunavir was recovered in the feces and urine, respectively. Unchanged darunavir accounted for approximately 41.2% and 7.7% of the administered dose in feces and urine, respectively. The terminal elimination half-life of darunavir was approximately 15 hours when co-administered with ritonavir. After intravenous administration, the clearance of darunavir, administered alone and co-administered with 100 mg twice daily ritonavir, was 32.8 L/h and 5.9 L/h, respectively.
Special Populations
Hepatic Impairment
Darunavir is primarily metabolized by the liver. The steady-state pharmacokinetic parameters of darunavir were similar after multiple dose co-administration of PREZISTA/ritonavir 600/100 mg twice daily to subjects with normal hepatic function (n=16), mild hepatic impairment (Child-Pugh Class A, n=8), and moderate hepatic impairment (Child-Pugh Class B, n=8). The effect of severe hepatic impairment on the pharmacokinetics of darunavir has not been evaluated [see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
Hepatitis B or Hepatitis C Virus Co-infection
The 48-week analysis of the data from Studies TMC114-C211 and TMC114-C214 in HIV-1-infected subjects indicated that hepatitis B and/or hepatitis C virus co-infection status had no apparent effect on the exposure of darunavir.
Renal Impairment
Results from a mass balance study with 14C-PREZISTA/ritonavir showed that approximately 7.7% of the administered dose of darunavir is excreted in the urine as unchanged drug. As darunavir and ritonavir are highly bound to plasma proteins, it is unlikely that they will be significantly removed by hemodialysis or peritoneal dialysis. Population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV-1-infected subjects with moderate renal impairment (CrCL between 30–60 mL/min, n=20). There are no pharmacokinetic data available in HIV-1-infected patients with severe renal impairment or end stage renal disease [see Use in Specific Populations (8.7)].
Gender
Population pharmacokinetic analysis showed higher mean darunavir exposure in HIV-1-infected females compared to males. This difference is not clinically relevant.
Race
Population pharmacokinetic analysis of darunavir in HIV-1-infected subjects indicated that race had no apparent effect on the exposure to darunavir.
Geriatric Patients
Population pharmacokinetic analysis in HIV-1-infected subjects showed that darunavir pharmacokinetics are not considerably different in the age range (18 to 75 years) evaluated in HIV-1-infected subjects (n=12, age greater than or equal to 65) [see Use in Specific Populations (8.5)].
Pediatric Patients
PREZISTA/ritonavir administered twice daily
The pharmacokinetics of darunavir in combination with ritonavir in 93 antiretroviral treatment-experienced HIV-1-infected pediatric subjects 3 to less than 18 years of age and weighing at least 10 kg showed that the administered weight-based dosages resulted in similar darunavir exposure when compared to the darunavir exposure achieved in treatment-experienced adults receiving PREZISTA/ritonavir 600/100 mg twice daily [see Dosage and Administration (2.5)].
PREZISTA/ritonavir administered once daily
The pharmacokinetics of darunavir in combination with ritonavir in 12 antiretroviral treatment-naïve HIV-1-infected pediatric subjects 12 to less than 18 years of age and weighing at least 40 kg receiving PREZISTA/ritonavir 800/100 mg once daily resulted in similar darunavir exposures when compared to the darunavir exposure achieved in treatment-naïve adults receiving PREZISTA/ritonavir 800/100 mg once daily [see Dosage and Administration (2.5)].
Based on population pharmacokinetic modeling and simulation, the proposed PREZISTA/ritonavir once daily dosing regimens for pediatric patients 3 to less than 12 years of age is predicted to result in similar darunavir exposures when compared to the darunavir exposures achieved in treatment-naïve adults receiving PREZISTA/ritonavir 800/100 mg once daily [see Dosage and Administration (2.5)].
The population pharmacokinetic parameters in pediatric subjects with PREZISTA/ritonavir administered once or twice daily are summarized in the table below:
Table 12: Population Pharmacokinetic Estimates of Darunavir Exposure (Trials TMC114-C230, TMC114-C212 and TMC114-C228) Following Administration of Doses in Tables 2 and 3 PREZISTA/ritonavir
once dailyPREZISTA/ritonavir
twice dailyTMC114-C228* Parameter TMC114-C230†
N=12TMC114-C212
N=7410 to less than 15 kg‡
N=1015 to less than 20 kg§
N=13N=number of subjects with data. - * Subjects may have contributed pharmacokinetic data to both the 10 kg to less than 15 kg weight group and the 15 kg to less than 20 kg weight group.
- † Summary statistics for population pharmacokinetic parameter estimates for DRV after administration of DRV/rtv at 800/100 mg once daily in treatment-naïve HIV-1 infected subjects from 12 to <18 years of age – Week-48 Analyses.
- ‡ Calculated from individual pharmacokinetic parameters estimated for Week 2 and Week 4, based on the Week 48 analysis that evaluated a darunavir dose of 20 mg/kg twice daily with ritonavir 3 mg/kg twice daily.
- § The 15 kg to less than 20 kg weight group received 380 mg (3.8 mL) PREZISTA oral suspension twice daily with 48 mg (0.6 mL) ritonavir oral solution twice daily in TMC114-C228. Calculated from individual pharmacokinetic parameters estimated for Week 2 post-dose adjustment visit; Week 24 and Week 48 based on the – Week 48 analysis that evaluated a darunavir dose of 380 mg twice daily.
- ¶ AUC24h is calculated as AUC12h*2.
AUC24h (ng∙h/mL) ¶ Mean ± Standard Deviation 84390 ± 23587 126377 ± 34356 137896 ± 51420 157760 ± 54080 Median
(Range)86741
(35527–123325)127340
(67054–230720)124044
(89688–261090)132698
(112310–294840)C0h (ng/mL) Mean ± Standard Deviation 2141 ± 865 3948 ± 1363 4510 ± 2031 4848 ± 2143 Median
(Range)2234
(542–3776)3888
(1836–7821)4126
(2456–9361)3927
(3046–10292)Pregnancy and Postpartum
The exposure to total darunavir and ritonavir after intake of PREZISTA/ritonavir 600/100 mg twice daily and PREZISTA/ritonavir 800/100 mg once daily as part of an antiretroviral regimen was generally lower during pregnancy compared with postpartum (see Table 13, Table 14 and Figure 1).
Table 13: Pharmacokinetic Results of Total Darunavir After Administration of PREZISTA/ritonavir at 600/100 mg Twice Daily as Part of an Antiretroviral Regimen, During the 2nd Trimester of Pregnancy, the 3rd Trimester of Pregnancy and Postpartum Pharmacokinetics of total darunavir
(mean ± standard deviation)2nd Trimester of pregnancy
(n=12)*3rd Trimester of pregnancy
(n=12)Postpartum (6–12 Weeks)
(n=12)- * n=11 for AUC24h
- † AUC24h is calculated as AUC12h*2.
Cmax, ng/mL 4668 ± 1097 5328 ± 1631 6659 ± 2364 AUC24h, ng.h/mL† 78740 ± 19194 91760 ± 34720 113780 ± 52680 Cmin, ng/mL 1922 ± 825 2661 ± 1269 2851 ± 2216 Table 14: Pharmacokinetic Results of Total Darunavir After Administration of PREZISTA/ritonavir at 800/100 mg Once Daily as Part of an Antiretroviral Regimen, During the 2nd Trimester of Pregnancy, the 3rd Trimester of Pregnancy and Postpartum Pharmacokinetics of total darunavir
(mean ± standard deviation)2nd Trimester of pregnancy
(n=17)3rd Trimester of pregnancy
(n=15)Postpartum (6–12 Weeks)
(n=16)Cmax, ng/mL 4964 ± 1505 5132 ± 1198 7310 ± 1704 AUC24h, ng.h/mL 62289 ± 16234 61112 ± 13790 92116 ± 29241 Cmin, ng/mL 1248 ± 542 1075 ± 594 1473 ± 1141 Due to an increase in the unbound fraction of darunavir during pregnancy compared to postpartum, unbound darunavir exposures were less reduced during pregnancy as compared to postpartum. Exposure reductions during pregnancy were greater for the once daily regimen as compared to the twice daily regimen (see Figure 1).
Figure 1: Pharmacokinetic Results (Within-Subject Comparison) of Total and Unbound Darunavir After Administration of PREZISTA/ritonavir at 600/100 mg Twice Daily or 800/100 mg Once Daily as Part of an Antiretroviral Regimen, During the 2nd and 3rd Trimester of Pregnancy Compared to Postpartum
Legend: 90% CI: 90% confidence interval; GMR: geometric mean ratio. Solid vertical line: ratio of 1.0; dotted vertical lines: reference lines of 0.8 and 1.25. 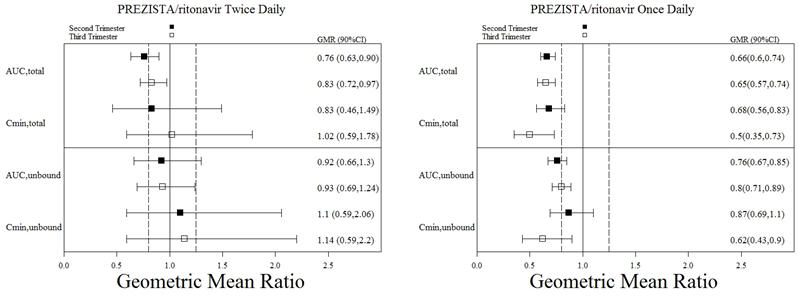
Drug Interactions
[See also Contraindications (4), Warnings and Precautions (5.5) and Drug Interactions (7).]
Darunavir co-administered with ritonavir is an inhibitor of CYP3A, CYP2D6, and P-gp. Co-administration of darunavir and ritonavir with drugs primarily metabolized by CYP3A and CYP2D6, or are transported by P-gp, may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and adverse events.
Darunavir and ritonavir are metabolized by CYP3A. In vitro data indicate that darunavir may be a P-gp substrate. Drugs that induce CYP3A activity would be expected to increase the clearance of darunavir and ritonavir, resulting in lowered plasma concentrations of darunavir and ritonavir. Co-administration of darunavir and ritonavir and other drugs that inhibit CYP3A or P-gp may decrease the clearance of darunavir and ritonavir and may result in increased plasma concentrations of darunavir and ritonavir.
Drug interaction studies were performed with darunavir and other drugs likely to be co-administered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of darunavir on the AUC, Cmax, and Cmin values are summarized in Table 15 (effect of other drugs on darunavir) and Table 16 (effect of darunavir on other drugs). For information regarding clinical recommendations, see Drug Interactions (7).
Several interaction studies have been performed with a dose other than the recommended dose of the co-administered drug or darunavir; however, the results are applicable to the recommended dose of the co-administered drug and/or darunavir.
Table 15: Drug Interactions: Pharmacokinetic Parameters for Darunavir in the Presence of Co-Administered Drugs Co-administered drug Dose/Schedule N PK LS Mean ratio (90% CI) of darunavir
Pharmacokinetic parameters with/without co-administered drug
no effect =1.00Co-administered Drug Darunavir/ritonavir Cmax AUC Cmin N = number of subjects with data - * q.d. = once daily
- † b.i.d. = twice daily
- ‡ The pharmacokinetic parameters of darunavir in this study were compared with the pharmacokinetic parameters following administration of PREZISTA/ritonavir 600/100 mg twice daily.
- § Ratio based on between-study comparison.
- ¶ The dose of simeprevir in this interaction study was 50 mg when co-administered in combination with PREZISTA/ritonavir compared to 150 mg once daily in the simeprevir alone treatment group.
- # Maximum number of subjects
- Þ q.o.d. = every other day
Co-administration with other HIV protease inhibitors Atazanavir 300 mg q.d.* 400/100 mg b.i.d. † 13 ↔ 1.02
(0.96–1.09)1.03
(0.94–1.12)1.01
(0.88–1.16)Indinavir 800 mg b.i.d. 400/100 mg b.i.d. 9 ↑ 1.11
(0.98–1.26)1.24
(1.09–1.42)1.44
(1.13–1.82)Lopinavir/ritonavir 400/100 mg b.i.d. 1200/100 mg b.i.d.‡ 14 ↓ 0.79
(0.67–0.92)0.62
(0.53–0.73)0.49
(0.39–0.63)533/133.3 mg b.i.d. 1200 mg b.i.d.‡ 15 ↓ 0.79
(0.64–0.97)0.59
(0.50–0.70)0.45
(0.38–0.52)Saquinavir hard gel capsule
1000 mg b.i.d. 400/100 mg b.i.d. 14 ↓ 0.83
(0.75–0.92)0.74
(0.63–0.86)0.58
(0.47–0.72)Co-administration with other HIV antiretrovirals Didanosine 400 mg q.d. 600/100 mg b.i.d. 17 ↔ 0.93
(0.86–1.00)1.01
(0.95–1.07)1.07
(0.95–1.21)Efavirenz 600 mg q.d. 300/100 mg b.i.d. 12 ↓ 0.85
(0.72–1.00)0.87
(0.75–1.01)0.69
(0.54–0.87)Etravirine 200 mg b.i.d. 600/100 mg b.i.d. 15 ↔ 1.11
(1.01–1.22)1.15
(1.05–1.26)1.02
(0.90–1.17)Nevirapine 200 mg b.i.d. 400/100 mg b.i.d. 8 ↑ 1.40 §
(1.14–1.73)1.24 §
(0.97–1.57)1.02 §
(0.79–1.32)Rilpivirine 150 mg q.d. 800/100 mg q.d. 15 ↔ 0.90
(0.81–1.00)0.89
(0.81–0.99)0.89
(0.68–1.16)Tenofovir disoproxil fumarate 300 mg q.d. 300/100 mg b.i.d. 12 ↑ 1.16
(0.94–1.42)1.21
(0.95–1.54)1.24
(0.90–1.69)Co-administration with HCV NS3-4A protease inhibitors Simeprevir 50 mg q.d. ¶ 800 mg q.d. 25# ↑ 1.04
(0.99–1.10)1.18
(1.11–1.25)1.31
(1.13–1.52)Co-administration with other drugs Artemether/lumefantrine 80/480 mg (6 doses at 0, 8, 24, 36, 48, and 60 hours) 600/100 mg b.i.d. 14 ↔ 1.00
(0.93–1.07)0.96
(0.90–1.03)0.87
(0.77–0.98)Carbamazepine 200 mg b.i.d. 600/100 mg b.i.d. 16 ↔ 1.04
(0.93–1.16)0.99
(0.90–1.08)0.85
(0.73–1.00)Clarithromycin 500 mg b.i.d. 400/100 mg b.i.d. 17 ↔ 0.83
(0.72–0.96)0.87
(0.75–1.01)1.01
(0.81–1.26)Ketoconazole 200 mg b.i.d. 400/100 mg b.i.d. 14 ↑ 1.21
(1.04–1.40)1.42
(1.23–1.65)1.73
(1.39–2.14)Omeprazole 20 mg q.d. 400/100 mg b.i.d. 16 ↔ 1.02
(0.95–1.09)1.04
(0.96–1.13)1.08
(0.93–1.25)Paroxetine 20 mg q.d. 400/100 mg b.i.d. 16 ↔ 0.97
(0.92–1.02)1.02
(0.95–1.10)1.07
(0.96–1.19)Pitavastatin 4 mg q.d. 800/100 mg q.d. 27 ↔ 1.06
(1.00–1.12)1.03
(0.95–1.12)NA Ranitidine 150 mg b.i.d. 400/100 mg b.i.d. 16 ↔ 0.96
(0.89–1.05)0.95
(0.90–1.01)0.94
(0.90–0.99)Rifabutin 150 mg q.o.d.Þ 600/100 mg b.i.d. 11 ↑ 1.42
(1.21–1.67)1.57
(1.28–1.93)1.75
(1.28–2.37)Sertraline 50 mg q.d. 400/100 mg b.i.d. 13 ↔ 1.01
(0.89–1.14)0.98
(0.84–1.14)0.94
(0.76–1.16)Table 16: Drug Interactions: Pharmacokinetic Parameters for Co-Administered Drugs in the Presence of PREZISTA/ritonavir Co-administered drug Dose/Schedule N PK LS Mean ratio (90% CI) of co-administered drug
pharmacokinetic parameters with/without darunavir
no effect =1.00Co-administered drug Darunavir/ritonavir Cmax AUC Cmin N = number of subjects with data;- = no information available
A cocktail study was conducted in 12 healthy volunteers to evaluate the effect of steady state pharmacokinetics of PREZISTA/ritonavir on the activity of CYP2D6 (using dextromethorphan as probe substrate), CYP2C9 (using warfarin as probe substrate), and CYP2C19 (using omeprazole as probe substrate). The pharmacokinetic results are shown in Table 16.- * q.d. = once daily
- † b.i.d. = twice daily
- ‡ The pharmacokinetic parameters of lopinavir in this study were compared with the pharmacokinetic parameters following administration of lopinavir/ritonavir 400/100 mg twice daily.
- § Noted as Cτ or C24 in the dolutegravir U.S. prescribing information
- ¶ The dose of simeprevir in this interaction study was 50 mg when co-administered in combination with PREZISTA/ritonavir compared to 150 mg once daily in the simeprevir alone treatment group.
- # Maximum number of subjects
- Þ ratio is for buprenorphine; mean Cmax and AUC24 for naloxone were comparable when buprenorphine/naloxone was administered with or without PREZISTA/ritonavir
- ß q.o.d. = every other day
- à In comparison to rifabutin 300 mg once daily.
Co-administration with other HIV protease inhibitors Atazanavir 300 mg q.d.* /100 mg ritonavir q.d. when administered alone 400/100 mg b.i.d. † 13 ↔ 0.89
(0.78–1.01)1.08
(0.94–1.24)1.52
(0.99–2.34)300 mg q.d. when administered with darunavir/ritonavir Indinavir 800 mg b.i.d. /100 mg ritonavir b.i.d. when administered alone 400/100 mg b.i.d. 9 ↑ 1.08
(0.95–1.22)1.23
(1.06–1.42)2.25
(1.63–3.10)800 mg b.i.d. when administered with darunavir/
ritonavir
Lopinavir/ritonavir 400/100 mg b.i.d.‡ 1200/100 mg b.i.d. 14 ↔ 0.98
(0.78–1.22)1.09
(0.86–1.37)1.23
(0.90–1.69)533/133.3 mg b.i.d.‡ 1200 mg b.i.d. 15 ↔ 1.11
(0.96–1.30)1.09
(0.96–1.24)1.13
(0.90–1.42)Saquinavir hard gel capsule 1000 mg b.i.d. /100 mg ritonavir b.i.d. when administered alone 400/100 mg b.i.d. 12 ↔ 0.94
(0.78–1.13)0.94
(0.76–1.17)0.82
(0.52–1.30)1000 mg b.i.d. when administered with darunavir/ritonavir Co-administration with other HIV antiretrovirals Didanosine 400 mg q.d. 600/100 mg b.i.d. 17 ↔ 0.84
(0.59–1.20)0.91
(0.75–1.10)- Dolutegravir 30 mg q.d 600/100 mg b.i.d. 15 ↓ 0.89
(0.83–0.97)0.78
(0.72–0.85)0.62§
(0.56–0.69)Dolutegravir 50 mg q.d. 600/100 mg b.i.d. with 200 mg b.i.d. etravirine 9 ↓ 0.88
(0.78–1.00)0.75
(0.69–0.81)0.63 §
(0.52–0.76)Efavirenz 600 mg q.d. 300/100 mg b.i.d. 12 ↑ 1.15
(0.97–1.35)1.21
(1.08–1.36)1.17
(1.01–1.36)Etravirine 100 mg b.i.d. 600/100 mg b.i.d. 14 ↓ 0.68
(0.57–0.82)0.63
(0.54–0.73)0.51
(0.44–0.61)Nevirapine 200 mg b.i.d. 400/100 mg b.i.d. 8 ↑ 1.18
(1.02–1.37)1.27
(1.12–1.44)1.47
(1.20–1.82)Rilpivirine 150 mg q.d. 800/100 mg q.d. 14 ↑ 1.79
(1.56–2.06)2.30
(1.98–2.67)2.78
(2.39–3.24)Tenofovir disoproxil fumarate 300 mg q.d. 300/100 mg b.i.d. 12 ↑ 1.24
(1.08–1.42)1.22
(1.10–1.35)1.37
(1.19–1.57)Maraviroc 150 mg b.i.d. 600/100 mg b.i.d. 12 ↑ 2.29
(1.46–3.59)4.05
(2.94–5.59)8.00
(6.35–10.1)600/100 mg b.i.d. with 200 mg b.i.d. etravirine 10 ↑ 1.77
(1.20–2.60)3.10
(2.57–3.74)5.27
(4.51–6.15)Co-administration with HCV NS3-4A protease inhibitors Simeprevir 50 mg q.d. ¶ 800/100 mg q.d. 25# ↑ 1.79
(1.55–2.06)2.59
(2.15–3.11)4.58
(3.54–5.92)Co-administration with other drugs Atorvastatin 40 mg q.d. when administered alone 300/100 mg b.i.d. 15 ↑ 0.56
(0.48–0.67)0.85
(0.76–0.97)1.81
(1.37–2.40)10 mg q.d. when administered with darunavir/ritonavir Artemether 80 mg single dose 600/100 mg b.i.d. 15 ↓ 0.85
(0.68–1.05)0.91
(0.78–1.06)- Dihydroartemisinin 15 ↑ 1.06
(0.82–1.39)1.12
(0.96–1.30)- Artemether artemether/lumefantrine 80/480 mg (6 doses at 0, 8, 24, 36, 48, and 60 hours) 600/100 mg b.i.d. 15 ↓ 0.82
(0.61–1.11)0.84
(0.69–1.02)0.97
(0.90–1.05)Dihydroartemisinin 15 ↓ 0.82
(0.66–1.01)0.82
(0.74–0.91)1.00
(0.82–1.22)Lumefantrine 15 ↑ 1.65
(1.49–1.83)2.75
(2.46–3.08)2.26
(1.92–2.67)Buprenorphine/Naloxone 8/2 mg to 16/4 mg q.d. 600/100 mg b.i.d. 17 ↔ 0.92 Þ
(0.79–1.08)0.89 Þ
(0.78–1.02)0.98 Þ
(0.82–1.16)Norbuprenorphine 17 ↑ 1.36
(1.06–1.74)1.46
(1.15–1.85)1.71
(1.29–2.27)Carbamazepine 200 mg b.i.d. 600/100 mg b.i.d. 16 ↑ 1.43
(1.34–1.53)1.45
(1.35–1.57)1.54
(1.41–1.68)Carbamazepine epoxide 16 ↓ 0.46
(0.43–0.49)0.46
(0.44–0.49)0.48
(0.45–0.51)Clarithromycin 500 mg b.i.d. 400/100 mg b.i.d. 17 ↑ 1.26
(1.03–1.54)1.57
(1.35–1.84)2.74
(2.30–3.26)Dextromethorphan 30 mg 600/100 mg b.i.d. 12 ↑ 2.27
(1.59–3.26)2.70
(1.80–4.05)- Dextrorphan ↓ 0.87
(0.77–0.98)0.96
(0.90–1.03)- Digoxin 0.4 mg 600/100 mg b.i.d. 8 ↑ 1.15
(0.89–1.48)1.36
(0.81–2.27)- Ethinyl estradiol (EE) Ortho-Novum 1/35
(35 µg EE /1 mg NE)600/100 mg b.i.d. 11 ↓ 0.68
(0.61–0.74)0.56
(0.50–0.63)0.38
(0.27–0.54)Norethindrone (NE) 11 ↓ 0.90
(0.83–0.97)0.86
(0.75–0.98)0.70
(0.51–0.97)Ketoconazole 200 mg b.i.d. 400/100 mg b.i.d. 15 ↑ 2.11
(1.81–2.44)3.12
(2.65–3.68)9.68
(6.44–14.55)R-Methadone 55–150 mg q.d. 600/100 mg b.i.d. 16 ↓ 0.76
(0.71–0.81)0.84
(0.78–0.91)0.85
(0.77–0.94)Omeprazole 40 mg single dose 600/100 mg b.i.d. 12 ↓ 0.66
(0.48–0.90)0.58
(0.50–0.66)- 5-hydroxy omeprazole ↓ 0.93
(0.71–1.21)0.84
(0.77–0.92)- Paroxetine 20 mg q.d. 400/100 mg b.i.d. 16 ↓ 0.64
(0.59–0.71)0.61
(0.56–0.66)0.63
(0.55–0.73)Pitavastatin 4 mg q.d. 800/100 mg q.d. 27 ↓ 0.96
(0.84–1.09)0.74
(0.69–0.80)NA Pravastatin 40 mg single dose 600/100 mg b.i.d. 14 ↑ 1.63
(0.95–2.82)1.81
(1.23–2.66)- Rifabutin 150 mg q.o.d. ß when administered with PREZISTA/ritonavir 600/100 mg b.i.d. à 11 ↑ 0.72
(0.55–0.93)0.93
(0.80–1.09)1.64
(1.48–1.81)25-O-desacetyl-rifabutin 300 mg q.d. when administered alone 11 ↑ 4.77
(4.04–5.63)9.81
(8.09–11.9)27.1
(22.2–33.2)Sertraline 50 mg q.d. 400/100 mg b.i.d. 13 ↓ 0.56
(0.49–0.63)0.51
(0.46–0.58)0.51
(0.45–0.57)Sildenafil 100 mg (single dose) administered alone 400/100 mg b.i.d. 16 ↑ 0.62
(0.55–0.70)0.97
(0.86–1.09)- 25 mg (single dose)when administered with darunavir/ritonavir S-warfarin 10 mg single dose 600/100 mg b.i.d. 12 ↓ 0.92
(0.86–0.97)0.79
(0.73–0.85)- 7-OH-S-warfarin 12 ↑ 1.42
(1.24–1.63)1.23
(0.97–1.57)- 12.4 Microbiology
Mechanism of Action
Darunavir is an inhibitor of the HIV-1 protease. It selectively inhibits the cleavage of HIV-1 encoded Gag-Pol polyproteins in infected cells, thereby preventing the formation of mature virus particles.
Antiviral Activity
Darunavir exhibits activity against laboratory strains and clinical isolates of HIV-1 and laboratory strains of HIV-2 in acutely infected T-cell lines, human peripheral blood mononuclear cells and human monocytes/macrophages with median EC50 values ranging from 1.2 to 8.5 nM (0.7 to 5.0 ng/mL). Darunavir demonstrates antiviral activity in cell culture against a broad panel of HIV-1 group M (A, B, C, D, E, F, G), and group O primary isolates with EC50 values ranging from less than 0.1 to 4.3 nM. The EC50 value of darunavir increases by a median factor of 5.4 in the presence of human serum. Darunavir did not show antagonism when studied in combination with the PIs amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, or tipranavir, the N(t)RTIs abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zalcitabine, or zidovudine, the NNRTIs delavirdine, rilpivirine, efavirenz, etravirine, or nevirapine, and the fusion inhibitor enfuvirtide.
Resistance
Cell Culture: HIV-1 isolates with a decreased susceptibility to darunavir have been selected in cell culture and obtained from subjects treated with PREZISTA/ritonavir. Darunavir-resistant virus derived in cell culture from wild-type HIV-1 had 21- to 88-fold decreased susceptibility to darunavir and developed 2 to 4 of the following amino acid substitutions S37D, R41E/T, K55Q, H69Q, K70E, T74S, V77I, or I85V in the protease. Selection in cell culture of darunavir resistant HIV-1 from nine HIV-1 strains harboring multiple PI resistance-associated mutations resulted in the overall emergence of 22 mutations in the protease gene, coding for amino acid substitutions L10F, V11I, I13V, I15V, G16E, L23I, V32I, L33F, S37N, M46I, I47V, I50V, F53L, L63P, A71V, G73S, L76V, V82I, I84V, T91A/S, and Q92R, of which L10F, V32I, L33F, S37N, M46I, I47V, I50V, L63P, A71V, and I84V were the most prevalent. These darunavir-resistant viruses had at least eight protease substitutions and exhibited 50- to 641-fold decreases in darunavir susceptibility with final EC50 values ranging from 125 nM to 3461 nM.
Clinical trials of PREZISTA/ritonavir in treatment-experienced subjects: In a pooled analysis of the 600/100 mg PREZISTA/ritonavir twice daily arms of trials TMC114-C213, TMC114-C202, TMC114-C215, and the control arms of etravirine trials TMC125-C206 and TMC125-C216, the amino acid substitutions V32I and I54L or M developed most frequently on PREZISTA/ritonavir in 41% and 25%, respectively, of the treatment-experienced subjects who experienced virologic failure, either by rebound or by never being suppressed (less than 50 copies/mL). Other substitutions that developed frequently in PREZISTA/ritonavir virologic failure isolates occurred at amino acid positions V11I, I15V, L33F, I47V, I50V, and L89V. These amino acid substitutions were associated with decreased susceptibility to darunavir; 90% of the virologic failure isolates had a greater than 7-fold decrease in susceptibility to darunavir at failure. The median darunavir phenotype (fold change from reference) of the virologic failure isolates was 4.3-fold at baseline and 85-fold at failure. Amino acid substitutions were also observed in the protease cleavage sites in the Gag polyprotein of some PREZISTA/ritonavir virologic failure isolates. In trial TMC114-C212 of treatment-experienced pediatric subjects, the amino acid substitutions V32I, I54L and L89M developed most frequently in virologic failures on PREZISTA/ritonavir.
In the 96-week as-treated analysis of the Phase 3 trial TMC114-C214, the percent of virologic failures (never suppressed, rebounders and discontinued before achieving suppression) was 21% (62/298) in the group of subjects receiving PREZISTA/ritonavir 600/100 mg twice daily compared to 32% (96/297) of subjects receiving lopinavir/ritonavir 400/100 mg twice daily. Examination of subjects who failed on PREZISTA/ritonavir 600/100 mg twice daily and had post-baseline genotypes and phenotypes showed that 7 subjects (7/43; 16%) developed PI substitutions on PREZISTA/ritonavir treatment resulting in decreased susceptibility to darunavir. Six of the 7 had baseline PI resistance-associated substitutions and baseline darunavir phenotypes greater than 7. The most common emerging PI substitutions in these virologic failures were V32I, L33F, M46I or L, I47V, I54L, T74P and L76V. These amino acid substitutions were associated with 59- to 839-fold decreased susceptibility to darunavir at failure. Examination of individual subjects who failed in the comparator arm on lopinavir/ritonavir and had post-baseline genotypes and phenotypes showed that 31 subjects (31/75; 41%) developed substitutions on lopinavir treatment resulting in decreased susceptibility to lopinavir (greater than 10-fold) and the most common substitutions emerging on treatment were L10I or F, M46I or L, I47V or A, I54V and L76V. Of the 31 lopinavir/ritonavir virologic failure subjects, 14 had reduced susceptibility (greater than 10-fold) to lopinavir at baseline.
In the 48-week analysis of the Phase 3 trial TMC114-C229, the number of virologic failures (including those who discontinued before suppression after Week 4) was 26% (75/294) in the group of subjects receiving PREZISTA/ritonavir 800/100 mg once daily compared to 19% (56/296) of subjects receiving PREZISTA/ritonavir 600/100 mg twice daily. Examination of isolates from subjects who failed on PREZISTA/ritonavir 800/100 mg once daily and had post-baseline genotypes showed that 8 subjects (8/60; 13%) had isolates that developed IAS-USA defined PI resistance-associated substitutions compared to 5 subjects (5/39; 13%) on PREZISTA/ritonavir 600/100 mg twice daily. Isolates from 2 subjects developed PI resistance associated substitutions associated with decreased susceptibility to darunavir; 1 subject isolate in the PREZISTA/ritonavir 800/100 mg once daily arm, developed substitutions V32I, M46I, L76V and I84V associated with a 24-fold decreased susceptibility to darunavir, and 1 subject isolate in the PREZISTA/ritonavir 600/100 mg twice daily arm developed substitutions L33F and I50V associated with a 40-fold decreased susceptibility to darunavir. In the PREZISTA/ritonavir 800/100 mg once daily and PREZISTA/ritonavir 600/100 mg twice daily groups, isolates from 7 (7/60; 12%) and 4 (4/42; 10%) virologic failures, respectively, developed decreased susceptibility to an NRTI included in the treatment regimen.
Clinical trials of PREZISTA/ritonavir in treatment-naïve subjects: In the 192-week as-treated analysis censoring those who discontinued before Week 4 of the Phase 3 trial TMC114-C211, the percentage of virologic failures (never suppressed, rebounders and discontinued before achieving suppression) was 22% (64/288) in the group of subjects receiving PREZISTA/ritonavir 800/100 mg once daily compared to 29% (76/263) of subjects receiving lopinavir/ritonavir 800/200 mg per day. In the PREZISTA/ritonavir arm, emergent PI resistance-associated substitutions were identified in 11 of the virologic failures with post-baseline genotypic data (n=43). However, none of the darunavir virologic failures had a decrease in darunavir susceptibility (greater than 7-fold change) at failure. In the comparator lopinavir/ritonavir arm, emergent PI resistance-associated substitutions were identified in 17 of the virologic failures with post-baseline genotypic data (n=53), but none of the lopinavir/ritonavir virologic failures had decreased susceptibility to lopinavir (greater than 10-fold change) at failure. The reverse transcriptase M184V substitution and/or resistance to emtricitabine, which was included in the fixed background regimen, was identified in 4 virologic failures from the PREZISTA/ritonavir arm and 7 virologic failures in the lopinavir/ritonavir arm.
Cross-resistance
Cross-resistance among PIs has been observed. Darunavir has a less than 10-fold decreased susceptibility in cell culture against 90% of 3309 clinical isolates resistant to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir and/or tipranavir showing that viruses resistant to these PIs remain susceptible to darunavir.
Darunavir-resistant viruses were not susceptible to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir or saquinavir in cell culture. However, six of nine darunavir-resistant viruses selected in cell culture from PI-resistant viruses showed a fold change in EC50 values less than 3 for tipranavir, indicative of limited cross-resistance between darunavir and tipranavir. In trials TMC114-C213, TMC114-C202, and TMC114-C215, 34% (64/187) of subjects in the PREZISTA/ritonavir arm whose baseline isolates had decreased susceptibility to tipranavir (tipranavir fold change greater than 3) achieved less than 50 copies/mL serum HIV-1 RNA levels at Week 96. Of the viruses isolated from subjects experiencing virologic failure on PREZISTA/ritonavir 600/100 mg twice daily (greater than 7-fold change), 41% were still susceptible to tipranavir and 10% were susceptible to saquinavir while less than 2% were susceptible to the other protease inhibitors (amprenavir, atazanavir, indinavir, lopinavir or nelfinavir).
In trial TMC114-C214, the 7 PREZISTA/ritonavir virologic failures with reduced susceptibility to darunavir at failure were also resistant to the approved PIs (fos)amprenavir, atazanavir, lopinavir, indinavir, and nelfinavir at failure. Six of these 7 were resistant to saquinavir and 5 were resistant to tipranavir. Four of these virologic failures were already PI-resistant at baseline.
Cross-resistance between darunavir and nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, fusion inhibitors, CCR5 co-receptor antagonists, or integrase inhibitors is unlikely because the viral targets are different.
Baseline Genotype/Phenotype and Virologic Outcome Analyses
Genotypic and/or phenotypic analysis of baseline virus may aid in determining darunavir susceptibility before initiation of PREZISTA/ritonavir 600/100 mg twice daily therapy. The effect of baseline genotype and phenotype on virologic response at 96 weeks was analyzed in as-treated analyses using pooled data from the Phase 2b trials (Trials TMC114-C213, TMC114-C202, and TMC114-C215) (n=439). The findings were confirmed with additional genotypic and phenotypic data from the control arms of etravirine trials TMC125-C206 and TMC125-C216 at Week 24 (n=591).
Diminished virologic responses were observed in subjects with 5 or more baseline IAS-defined primary protease inhibitor resistance-associated substitutions (D30N, V32I, L33F, M46I/L, I47A/V, G48V, I50L/V, I54L/M, L76V, V82A/F/L/S/T, I84V, N88S, L90M) (see Table 17).
Table 17: Response to PREZISTA/ritonavir 600/100 mg Twice Daily by Baseline Number of IAS-Defined Primary PI Resistance-Associated Substitutions: As-treated Analysis of Trials TMC114-C213, TMC114-C202, and TMC114-C215 # IAS-defined primary PI substitutions Proportion of subjects with <50 copies/mL at Week 96
N=439Overall de novo ENF Re-used/No ENF ENF=enfuvirtide All 44% (192/439) 54% (61/112) 40% (131/327) 0 – 4 50% (162/322) 58% (49/85) 48% (113/237) 5 22% (16/74) 47% (9/19) 13% (7/55) ≥6 9% (3/32) 17% (1/6) 8% (2/26) IAS Primary PI Substitutions (2008): D30N, V32I, L33F, M46I/L, I47A/V, G48V, I50L/V, I54L/M, L76V, V82A/F/L/S/T, I84V, N88S, L90M
The presence at baseline of two or more of the substitutions V11I, V32I, L33F, I47V, I50V, I54L or M, T74P, L76V, I84V or L89V was associated with a decreased virologic response to PREZISTA/ritonavir. In subjects not taking enfuvirtide de novo, the proportion of subjects achieving viral load less than 50 plasma HIV-1 RNA copies/mL at 96 weeks was 59%, 29%, and 12% when the baseline genotype had 0–1, 2 and greater than or equal to 3 of these substitutions, respectively.
Baseline darunavir phenotype (shift in susceptibility relative to reference) was shown to be a predictive factor of virologic outcome. Response rates assessed by baseline darunavir phenotype are shown in Table 18. These baseline phenotype groups are based on the select patient populations in the trials TMC114-C213, TMC114-C202, and TMC114-C215, and are not meant to represent definitive clinical susceptibility breakpoints for PREZISTA/ritonavir. The data are provided to give clinicians information on the likelihood of virologic success based on pre-treatment susceptibility to darunavir.
Table 18: Response (HIV-1 RNA <50 copies/mL at Week 96) to PREZISTA/ritonavir 600/100 mg Twice Daily by Baseline Darunavir Phenotype and by Use of Enfuvirtide: As-treated Analysis of Trials TMC114-C213, TMC114-C202, and TMC114-C215 Baseline DRV phenotype Proportion of subjects with <50 copies/mL at Week 96
N=417All de novo ENF Re-used/No ENF ENF=enfuvirtide Overall 175/417 (42%) 61/112 (54%) 131/327 (40%) 0 – 7 148/270 (55%) 44/65 (68%) 104/205 (51%) >7 – 20 16/53 (30%) 7/17 (41%) 9/36 (25%) >20 11/94 (12%) 6/23 (26%) 5/71 (7%) -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Darunavir was evaluated for carcinogenic potential by oral gavage administration to mice and rats up to 104 weeks. Daily doses of 150, 450 and 1000 mg/kg were administered to mice and doses of 50, 150 and 500 mg/kg was administered to rats. A dose-related increase in the incidence of hepatocellular adenomas and carcinomas were observed in males and females of both species as well as an increase in thyroid follicular cell adenomas in male rats. The observed hepatocellular findings in rodents are considered to be of limited relevance to humans. Repeated administration of darunavir to rats caused hepatic microsomal enzyme induction and increased thyroid hormone elimination, which predispose rats, but not humans, to thyroid neoplasms. At the highest tested doses, the systemic exposures to darunavir (based on AUC) were between 0.4- and 0.7-fold (mice) and 0.7- and 1-fold (rats), relative to those observed in humans at the recommended therapeutic doses (600/100 mg twice daily or 800/100 mg once daily).
Darunavir was not mutagenic or genotoxic in a battery of in vitro and in vivo assays including bacterial reserve mutation (Ames), chromosomal aberration in human lymphocytes and in vivo micronucleus test in mice.
-
14 CLINICAL STUDIES
14.1 Description of Adult Clinical Trials
The evidence of efficacy of PREZISTA/ritonavir is based on the analyses of 192-week data from a randomized, controlled open-label Phase 3 trial in treatment-naïve (TMC114-C211) HIV-1-infected adult subjects and 96-week data from a randomized, controlled, open-label Phase 3 trial in antiretroviral treatment-experienced (TMC114-C214) HIV-1-infected adult subjects. In addition, 96-week data are included from 2 randomized, controlled Phase 2b trials, TMC114-C213 and TMC114-C202, in antiretroviral treatment-experienced HIV-1-infected adult subjects.
14.2 Treatment-Naïve Adult Subjects
TMC114-C211
TMC114-C211 is a randomized, controlled, open-label Phase 3 trial comparing PREZISTA/ritonavir 800/100 mg once daily versus lopinavir/ritonavir 800/200 mg per day (given as a twice daily or as a once daily regimen) in antiretroviral treatment-naïve HIV-1-infected adult subjects. Both arms used a fixed background regimen consisting of tenofovir disoproxil fumarate 300 mg once daily (TDF) and emtricitabine 200 mg once daily (FTC).
HIV-1-infected subjects who were eligible for this trial had plasma HIV-1 RNA greater than or equal to 5000 copies/mL. Randomization was stratified by screening plasma viral load (HIV-1 RNA less than 100,000 copies/mL or greater than or equal to 100,000 copies/mL) and screening CD4+ cell count (less than 200 cells/mm3 or greater than or equal to 200 cells/mm3). Virologic response was defined as a confirmed plasma HIV-1 RNA viral load less than 50 copies/mL. Analyses included 689 subjects in trial TMC114-C211 who had completed 192 weeks of treatment or discontinued earlier.
Demographics and baseline characteristics were balanced between the PREZISTA/ritonavir arm and the lopinavir/ritonavir arm (see Table 19). Table 19 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and subjects in the lopinavir/ritonavir 800/200 mg per day arm in trial TMC114-C211.
Table 19: Demographic and Baseline Characteristics of Subjects in Trial TMC114-C211 PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC
N=343lopinavir/ritonavir 800/200 mg per day + TDF/FTC
N=346FTC=emtricitabine; TDF=tenofovir disoproxil fumarate Demographic characteristics Median age (years) (range, years) 34 (18–70) 33 (19–68) Sex Male 70% 70% Female 30% 30% Race White 40% 45% Black 23% 21% Hispanic 23% 22% Asian 13% 11% Baseline characteristics Mean baseline plasma HIV-1 RNA (log10 copies/mL) 4.86 4.84 Median baseline CD4+ cell count (cells/mm3)
(range, cells/mm3)228 (4–750) 218 (2–714) Percentage of patients with baseline viral load ≥100,000 copies/mL 34% 35% Percentage of patients with baseline CD4+ cell count <200 cells/mm3 41% 43% Week 192 outcomes for subjects on PREZISTA/ritonavir 800/100 mg once daily from trial TMC114-C211 are shown in Table 20.
Table 20: Virologic Outcome of Randomized Treatment of Trial TMC114-C211 at 192 Weeks PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC
N=343lopinavir/ritonavir 800/200 mg per day + TDF/FTC
N=346N = total number of subjects with data; FTC=emtricitabine; TDF=tenofovir disoproxil fumarate - * 95% CI: 1.9; 16.1
- † Includes patients who discontinued prior to Week 192 for lack or loss of efficacy and patients who are ≥50 copies in the 192-week window and patients who had a change in their background regimen that was not permitted by the protocol.
- ‡ Window 186–198 Weeks.
- § Includes patients who discontinued due to adverse event or death at any time point from Day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
- ¶ Other includes: withdrew consent, loss to follow-up, etc., if the viral load at the time of discontinuation was <50 copies/mL
Virologic success HIV-1 RNA <50 copies/mL 70%* 61% Virologic failure† 12% 15% No virologic data at Week 192 window‡ Reasons Discontinued trial due to adverse event or death§ 5% 13% Discontinued trial for other reasons¶ 13% 12% Missing data during window‡ but on trial <1% 0% In trial TMC114-C211 at 192 weeks of treatment, the median increase from baseline in CD4+ cell counts was 258 cells/mm3 in the PREZISTA/ritonavir 800/100 mg once daily arm and 263 cells/mm3 in the lopinavir/ritonavir 800/200 mg per day arm. Of the PREZISTA/ritonavir subjects with a confirmed virologic response of <50 copies/mL at Week 48, 81% remained undetectable at Week 192 versus 68% with lopinavir/ritonavir. In the 192 week analysis, statistical superiority of the PREZISTA/ritonavir regimen over the lopinavir/ritonavir regimen was demonstrated for both ITT and OP populations.
14.3 Treatment-Experienced Adult Subjects
TMC114-C229
TMC114-C229 is a randomized, open-label trial comparing PREZISTA/ritonavir 800/100 mg once daily to PREZISTA/ritonavir 600/100 mg twice daily in treatment-experienced HIV-1-infected patients with screening genotype resistance test showing no darunavir resistance associated substitutions (i.e. V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, L89V) and a screening viral load of greater than 1,000 HIV-1 RNA copies/mL. Both arms used an optimized background regimen consisting of greater than or equal to 2 NRTIs selected by the investigator.
HIV-1-infected subjects who were eligible for this trial were on a highly active antiretroviral therapy regimen (HAART) for at least 12 weeks. Virologic response was defined as a confirmed plasma HIV-1 RNA viral load less than 50 copies/mL. Analyses included 590 subjects who had completed 48 weeks of treatment or discontinued earlier.
Table 21 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm in trial TMC114-C229. No imbalances between the 2 arms were noted.
Table 21: Demographic and Baseline Characteristics of Subjects in Trial TMC114-C229 PREZISTA/ritonavir 800/100 mg once daily + OBR
N=294PREZISTA/ritonavir 600/100 mg twice daily + OBR
N=296OBR=optimized background regimen - * Based on phenotype (Antivirogram®)
- † Johnson VA, Brun-Vézinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2008. Top HIV Med 2008; 16(5): 138–145
- ‡ Only counting ARVs, excluding low-dose ritonavir
Demographic characteristics Median age (years) (range, years) 40 (18–70) 40 (18–77) Sex Male 61% 67% Female 39% 33% Race White 35% 37% Black 28% 24% Hispanic 16% 20% Asian 16% 14% Baseline characteristics Mean baseline plasma HIV-1 RNA (log10 copies/mL) 4.19 4.13 Median baseline CD4+ cell count (cells/mm3) (range, cells/mm3) 219 (24–1306) 236 (44–864) Percentage of patients with baseline viral load ≥100,000 copies/mL 13% 11% Percentage of patients with baseline CD4+ cell count <200 cells/mm3 43% 39% Median darunavir fold change (range)* 0.50 (0.1–1.8) 0.50 (0.1–1.9) Median number of resistance-associated†: PI mutations 3 4 NNRTI mutations 2 1 NRTI mutations 1 1 Percentage of subjects susceptible to all available PIs at baseline 88% 86% Percentage of subjects with number of baseline primary protease inhibitor mutations†: 0 84% 84% 1 8% 9% 2 5% 4% ≥3 3% 2% Median number of ARVs previously used‡: NRTIs 3 3 NNRTIs 1 1 PIs (excluding low-dose ritonavir) 1 1 Week 48 outcomes for subjects on PREZISTA/ritonavir 800/100 mg once daily from trial TMC114-C229 are shown in Table 22.
Table 22: Virologic Outcome of Randomized Treatment of Trial TMC114-C229 at 48 Weeks PREZISTA/ritonavir 800/100 mg once daily + OBR
N=294PREZISTA/ritonavir 600/100 mg twice daily + OBR
N=296N = total number of subjects with data; OBR=optimized background regimen - * Includes patients who discontinued prior to Week 48 for lack or loss of efficacy, patients who are ≥50 copies in the 48-week window, patients who had a change in their background regimen that was not permitted in the protocol (provided the switch occurred before the earliest onset of an AE leading to permanent stop of trial medication) and patients who discontinued for reasons other than AEs/death and lack or loss of efficacy (provided their last available viral load was detectable (HIV RNA ≥50 copies/mL).
- † Window 42–54 Weeks
- ‡ Patients who discontinued due to adverse event or death at any time point from Day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
- § Other includes: withdrew consent, loss to follow-up, etc., if the viral load at the time of discontinuation was <50 copies/mL.
Virologic success HIV-1 RNA <50 copies/mL 69% 69% Virologic failure* 26% 23% No virologic data at Week 48 window† Reasons Discontinued trial due to adverse event or death‡ 3% 4% Discontinued trial for other reasons§ 2% 3% Missing data during window† but on trial 0% <1% The mean increase from baseline in CD4+ cell counts was comparable for both treatment arms (108 cells/mm3 and 112 cells/mm3 in the PREZISTA/ritonavir 800/100 mg once daily arm and the PREZISTA/ritonavir 600/100 mg twice daily arm, respectively).
TMC114-C214
TMC114-C214 is a randomized, controlled, open-label Phase 3 trial comparing PREZISTA/ritonavir 600/100 mg twice daily versus lopinavir/ritonavir 400/100 mg twice daily in antiretroviral treatment-experienced, lopinavir/ritonavir-naïve HIV-1-infected adult subjects. Both arms used an optimized background regimen consisting of at least 2 antiretrovirals (NRTIs with or without NNRTIs).
HIV-1-infected subjects who were eligible for this trial had plasma HIV-1 RNA greater than 1000 copies/mL and were on a highly active antiretroviral therapy regimen (HAART) for at least 12 weeks. Virologic response was defined as a confirmed plasma HIV-1 RNA viral load less than 400 copies/mL. Analyses included 595 subjects in trial TMC114-C214 who had completed 96 weeks of treatment or discontinued earlier.
Demographics and baseline characteristics were balanced between the PREZISTA/ritonavir arm and the lopinavir/ritonavir arm (see Table 23). Table 23 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm and subjects in the lopinavir/ritonavir 400/100 mg twice daily arm in trial TMC114-C214.
Table 23: Demographic and Baseline Characteristics of Subjects in Trial TMC114-C214 PREZISTA/ritonavir 600/100 mg twice daily + OBR
N=298lopinavir/ritonavir 400/100 mg twice daily + OBR
N=297OBR=optimized background regimen - * Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: Fall 2006. Top HIV Med 2006; 14(3): 125–130
- † Only counting ARVs, excluding low-dose ritonavir
- ‡ Based on phenotype (Antivirogram®)
- § Commercially available PIs at the time of trial enrollment
Demographic characteristics Median age (years) (range, years) 40 (18–68) 41 (22–76) Sex Male 77% 81% Female 23% 19% Race White 54% 57% Black 18% 17% Hispanic 15% 15% Asian 9% 9% Baseline characteristics Mean baseline plasma HIV-1 RNA (log10 copies/mL) 4.33 4.28 Median baseline CD4+ cell count (cells/mm3) (range, cells/mm3) 235 (3–831) 230 (2–1096) Percentage of patients with baseline viral load ≥100,000 copies/mL 19% 17% Percentage of patients with baseline CD4+ cell count <200 cells/mm3 40% 40% Median darunavir fold change (range) 0.60 (0.10–37.40) 0.60 (0.1–43.8) Median lopinavir fold change (range) 0.70 (0.40–74.40) 0.80 (0.30–74.50) Median number of resistance-associated*: PI mutations 4 4 NNRTI mutations 1 1 NRTI mutations 2 2 Percentage of subjects with number of baseline primary protease inhibitor mutations*: ≤1 78% 80% 2 8% 9% ≥3 13% 11% Median number of ARVs previously used†: NRTIs 4 4 NNRTIs 1 1 PIs (excluding low-dose ritonavir) 1 1 Percentage of subjects resistant‡ to all available§ PIs at baseline, excluding darunavir 2% 3% Week 96 outcomes for subjects on PREZISTA/ritonavir 600/100 mg twice daily from trial TMC114-C214 are shown in Table 24.
Table 24: Virologic Outcome of Randomized Treatment of Trial TMC114-C214 at 96 Weeks PREZISTA /ritonavir 600/100 mg twice daily + OBR
N=298lopinavir/ritonavir 400/100 mg twice daily + OBR
N=297N = total number of subjects with data; OBR=optimized background regimen - * Includes patients who discontinued prior to Week 96 for lack or loss of efficacy and patients who are ≥50 copies in the 96-week window and patients who had a change in their OBR that was not permitted by the protocol.
- † Window 90–102 Weeks
- ‡ Includes patients who discontinued due to adverse event or death at any time point from Day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
- § Other includes: withdrew consent, loss to follow-up, etc., if the viral load at the time of discontinuation was <50 copies/mL.
Virologic success HIV-1 RNA <50 copies/mL 58% 52% Virologic failure* 26% 33% No virologic data at Week 96 window† Reasons Discontinued trial due to adverse event or death‡ 7% 8% Discontinued trial for other reasons§ 8% 7% Missing data during window† but on trial 1% <1% In trial TMC114-C214 at 96 weeks of treatment, the median increase from baseline in CD4+ cell counts was 81 cells/mm3 in the PREZISTA/ritonavir 600/100 mg twice daily arm and 93 cells/mm3 in the lopinavir/ritonavir 400/100 mg twice daily arm.
TMC114-C213 and TMC114-C202
TMC114-C213 and TMC114-C202 are randomized, controlled, Phase 2b trials in adult subjects with a high level of PI resistance consisting of 2 parts: an initial partially-blinded, dose-finding part and a second long-term part in which all subjects randomized to PREZISTA/ritonavir received the recommended dose of 600/100 mg twice daily.
HIV-1-infected subjects who were eligible for these trials had plasma HIV-1 RNA greater than 1000 copies/mL, had prior treatment with PI(s), NNRTI(s) and NRTI(s), had at least one primary PI mutation (D30N, M46I/L, G48V, I50L/V, V82A/F/S/T, I84V, L90M) at screening, and were on a stable PI-containing regimen at screening for at least 8 weeks. Randomization was stratified by the number of PI mutations, screening viral load, and the use of enfuvirtide.
The virologic response rate was evaluated in subjects receiving PREZISTA/ritonavir plus an OBR versus a control group receiving an investigator-selected PI(s) regimen plus an OBR. Prior to randomization, PI(s) and OBR were selected by the investigator based on genotypic resistance testing and prior ARV history. The OBR consisted of at least 2 NRTIs with or without enfuvirtide. Selected PI(s) in the control arm included: lopinavir in 36%, (fos)amprenavir in 34%, saquinavir in 35% and atazanavir in 17%; 98% of control subjects received a ritonavir boosted PI regimen out of which 23% of control subjects used dual-boosted PIs. Approximately 47% of all subjects used enfuvirtide, and 35% of the use was in subjects who were ENF-naïve. Virologic response was defined as a decrease in plasma HIV-1 RNA viral load of at least 1 log10 versus baseline.
In the pooled analysis for TMC114-C213 and TMC114-C202, demographics and baseline characteristics were balanced between the PREZISTA/ritonavir arm and the comparator PI arm (see Table 25). Table 25 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm and subjects in the comparator PI arm in the pooled analysis of trials TMC114-C213 and TMC114-C202.
Table 25: Demographic and Baseline Characteristics of Subjects in the Trials TMC114-C213 and TMC114-C202 (Pooled Analysis) PREZISTA/ritonavir 600/100 mg twice daily + OBR Comparator PI(s) + OBR N=131 N=124 OBR=optimized background regimen - * Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: Fall 2006. Top HIV Med 2006; 14(3): 125–130
- † Based on phenotype (Antivirogram®)
- ‡ Commercially available PIs at the time of trial enrollment
Demographic characteristics Median age (years) (range, years) 43 (27–73) 44 (25–65) Sex Male 89% 88% Female 11% 12% Race White 81% 73% Black 10% 15% Hispanic 7% 8% Baseline characteristics Mean baseline plasma HIV-1 RNA (log10 copies/mL) 4.61 4.49 Median baseline CD4+ cell count (cells/mm3) (range, cells/mm3) 153 (3–776) 163 (3–1274) Percentage of patients with baseline viral load >100,000 copies/mL 24% 29% Percentage of patients with baseline CD4+ cell count <200 cells/mm3 67% 58% Median darunavir fold change 4.3 3.3 Median number of resistance-associated*: PI mutations 12 12 NNRTI mutations 1 1 NRTI mutations 5 5 Percentage of subjects with number of baseline primary protease inhibitor mutations*: ≤1 8% 9% 2 22% 21% ≥3 70% 70% Median number of ARVs previously used†: NRTIs 6 6 NNRTIs 1 1 PIs (excluding low-dose ritonavir) 5 5 Percentage of subjects resistant† to all available‡ PIs at baseline, excluding tipranavir and darunavir 63% 61% Percentage of subjects with prior use of enfuvirtide
20% 17% Week 96 outcomes for subjects on the recommended dose PREZISTA/ritonavir 600/100 mg twice daily from the pooled trials TMC114-C213 and TMC114-C202 are shown in Table 26.
Table 26: Outcomes of Randomized Treatment Through Week 96 of the Trials TMC114-C213 and TMC114-C202 (Pooled Analysis) Randomized trials
TMC114-C213 and TMC114-C202PREZISTA/ritonavir 600/100 mg twice daily + OBR Comparator PI(s) + OBR N=131 N=124 OBR=optimized background regimen - * Subjects who did not achieve at least a confirmed 0.5 log10 HIV-1 RNA drop from baseline at Week 12
- † Subjects with an initial response (confirmed 1 log10 drop in viral load), but without a confirmed 1 log10 drop in viral load at Week 96
- ‡ Subjects who never reached a confirmed 1 log10 drop in viral load before Week 96
Virologic responders confirmed at least 1 log10 HIV-1 RNA below baseline through Week 96 (<50 copies/mL at Week 96) 57% (39%) 10% (9%) Virologic failures 29% 80% Lack of initial response* 8% 53% Rebounder† 17% 19% Never suppressed‡ 4% 8% Death or discontinuation due to adverse events 9% 3% Discontinuation due to other reasons 5% 7% In the pooled trials TMC114-C213 and TMC114-C202 through 48 weeks of treatment, the proportion of subjects with HIV-1 RNA less than 400 copies/mL in the arm receiving PREZISTA/ritonavir 600/100 mg twice daily compared to the comparator PI arm was 55.0% and 14.5%, respectively. In addition, the mean changes in plasma HIV-1 RNA from baseline were –1.69 log10 copies/mL in the arm receiving PREZISTA/ritonavir 600/100 mg twice daily and –0.37 log10 copies/mL for the comparator PI arm. The mean increase from baseline in CD4+ cell counts was higher in the arm receiving PREZISTA/ritonavir 600/100 mg twice daily (103 cells/mm3) than in the comparator PI arm (17 cells/mm3).
14.4 Pediatric Patients
The pharmacokinetic profile, safety and antiviral activity of PREZISTA/ritonavir were evaluated in 3 randomized, open-label, multicenter studies.
TMC114-C212
Treatment-experienced pediatric subjects between the ages of 6 and less than 18 years and weighing at least 20 kg were stratified according to their weight (greater than or equal to 20 kg to less than 30 kg, greater than or equal to 30 kg to less than 40 kg, greater than or equal to 40 kg) and received PREZISTA tablets with either ritonavir capsules or oral solution plus background therapy consisting of at least two non-protease inhibitor antiretroviral drugs. Eighty patients were randomized and received at least one dose of PREZISTA/ritonavir. Pediatric subjects who were at risk of discontinuing therapy due to intolerance of ritonavir oral solution (e.g., taste aversion) were allowed to switch to the capsule formulation. Of the 44 pediatric subjects taking ritonavir oral solution, 23 subjects switched to the 100 mg capsule formulation and exceeded the weight-based ritonavir dose without changes in observed safety.
The 80 randomized pediatric subjects had a median age of 14 (range 6 to less than 18 years), and were 71% male, 54% Caucasian, 30% Black, 9% Hispanic and 8% other. The mean baseline plasma HIV-1 RNA was 4.64 log10 copies/mL, and the median baseline CD4+ cell count was 330 cells/mm3 (range: 6 to 1505 cells/mm3). Overall, 38% of pediatric subjects had baseline plasma HIV-1 RNA ≥100,000 copies/mL. Most pediatric subjects (79%) had previous use of at least one NNRTI and 96% of pediatric subjects had previously used at least one PI.
Seventy-seven pediatric subjects (96%) completed the 24-week period. Of the patients who discontinued, one patient discontinued treatment due to an adverse event. An additional 2 patients discontinued for other reasons, one patient due to compliance and another patient due to relocation.
The proportion of pediatric subjects with HIV-1 RNA less than 400 copies/mL and less than 50 copies/mL was 64% and 50%, respectively. The mean increase in CD4+ cell count from baseline was 117 cells/mm3.
TMC114-C228
Treatment-experienced pediatric subjects between the ages of 3 and less than 6 years and weighing greater than or equal to 10 kg to less than 20 kg received PREZISTA oral suspension with ritonavir oral solution plus background therapy consisting of at least two active non-protease inhibitor antiretroviral drugs. Twenty-one subjects received at least one dose of PREZISTA/ritonavir.
The 21 subjects had a median age of 4.4 years (range 3 to less than 6 years), and were 48% male, 57% Black, 29%, Caucasian and 14% other. The mean baseline plasma HIV-1 was 4.34 log10 copies/mL, the median baseline CD4+ cell count was 927 × 106 cells/L (range: 209 to 2,429 × 106 cells/L) and the median baseline CD4+ percentage was 27.7% (range: 15.6% to 51.1%). Overall, 24% of subjects had a baseline plasma HIV-1 RNA greater than or equal to 100,000 copies/mL. All subjects had used greater than or equal to 2 NRTIs, 62% of subjects had used greater than or equal to 1 NNRTI and 76% had previously used at least one HIV PI.
Twenty subjects (95%) completed the 48 week period. One subject prematurely discontinued treatment due to vomiting assessed as related to ritonavir.
The proportion of subjects with HIV-1 RNA less than 50 copies/mL at Week 48 was 71%. The mean increase in CD4+ percentage from baseline was 4%. The mean change in CD4+ cell count from baseline was 187 × 106 cells/L.
TMC114-C230
Treatment-naïve pediatric subjects between the ages of 12 and less than 18 years and weighing at least 40 kg received the adult recommended dose of PREZISTA/ritonavir 800/100 mg once daily plus background therapy consisting of at least two non-protease inhibitor antiretroviral drugs.
The 12 randomized pediatric subjects had a median age of 14.4 years (range 12.6 to 17.3 years), and were 33.3% male, 58.3% Caucasian and 41.7% Black. The mean baseline plasma HIV-1 RNA was 4.72 log10 copies/mL, and the median baseline CD4+ cell count was 282 cells/mm3 (range: 204 to 515 cells/mm3). Overall, 41.7% of pediatric subjects had baseline plasma HIV-1 RNA ≥100,000 copies/mL.
All subjects completed the 48 week treatment period.
The proportion of subjects with HIV-1 RNA less than 50 copies/mL and less than 400 copies/mL was 83.3% and 91.7%, respectively. The mean increase in CD4+ cell count from baseline was 221 × 106 cells/L.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PREZISTA (darunavir) 100 mg per mL oral suspension is a white to off-white opaque liquid supplied in amber-colored multiple-dose bottles containing darunavir ethanolate equivalent to 100 mg of darunavir per mL packaged with a 6 mL oral dosing syringe with 0.2 mL gradations.
PREZISTA (darunavir) 75 mg tablets are supplied as white, caplet-shaped, film-coated tablets containing darunavir ethanolate equivalent to 75 mg of darunavir per tablet. Each tablet is debossed with "75" on one side and "TMC" on the other side.
PREZISTA (darunavir) 150 mg tablets are supplied as white, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 150 mg of darunavir per tablet. Each tablet is debossed with "150" on one side and "TMC" on the other side.
PREZISTA (darunavir) 600 mg tablets are supplied as orange, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 600 mg of darunavir per tablet. Each tablet is debossed with "600MG" on one side and "TMC" on the other side.
PREZISTA (darunavir) 800 mg tablets are supplied as dark red, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 800 mg of darunavir per tablet. Each tablet is debossed with "800" on one side and "T" on the other side.
PREZISTA is packaged in bottles in the following configuration:
- 100 mg/mL oral suspension – 200 mL bottles (NDC: 59676-565-01)
- 75 mg tablets — bottles of 480 (NDC: 59676-563-01)
- 150 mg tablets — bottles of 240 (NDC: 59676-564-01)
- 600 mg tablets — bottles of 60 (NDC: 59676-562-01)
- 800 mg tablets — bottles of 30 (NDC: 59676-566-30)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instruction for Use).
Instructions for Use
Advise patients to take PREZISTA and ritonavir with food every day on a regular dosing schedule, as missed doses can result in development of resistance. PREZISTA must always be used with ritonavir in combination with other antiretroviral drugs. Advise patients not to alter the dose of either PREZISTA or ritonavir, discontinue ritonavir, or discontinue therapy with PREZISTA without consulting their physician [see Dosage and Administration (2)].
Hepatotoxicity
Inform patients that drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) has been reported with PREZISTA co-administered with 100 mg of ritonavir. Advise patients about the signs and symptoms of liver problems [see Warnings and Precautions (5.2)].
Severe Skin Reactions
Inform patients that skin reactions ranging from mild to severe, including Stevens-Johnson Syndrome, drug rash with eosinophilia and systemic symptoms, and toxic epidermal necrolysis, have been reported with PREZISTA co-administered with 100 mg of ritonavir. Advise patients to discontinue PREZISTA/ritonavir immediately if signs or symptoms of severe skin reactions develop. These can include but are not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and/or eosinophilia [see Warnings and Precautions (5.3)].
Drug Interactions
PREZISTA/ritonavir may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John's wort [see Contraindications (4), Warnings and Precautions (5.4, 5.5) and Drug Interactions (7)].
Contraception
Instruct patients receiving combined hormonal contraception or the progestin only pill to use an effective alternative (non-hormonal) contraceptive method or add a barrier method during therapy with PREZISTA/ritonavir because hormonal levels may decrease [see Drug Interactions (7.3) and Use in Specific Populations (8.3)].
Fat Redistribution
Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy, including PREZISTA/ritonavir, and that the cause and long-term health effects of these conditions are not known at this time [see Warnings and Precautions (5.7)].
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.8)].
Pregnancy Registry
Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to PREZISTA [see Use in Specific Populations (8.1)].
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTION
Product of Ireland
Manufactured by:
PREZISTA oral suspension
Janssen Pharmaceutica, NV
Beerse, BelgiumPREZISTA tablets
Janssen Ortho LLC,
Gurabo,
PR 00778Or
Janssen Cilag SpA,
Latina,
ITManufactured for:
Janssen Therapeutics,
Division of Janssen Products, LP,
Titusville NJ 08560PREZISTA® is a registered trademark of Janssen Pharmaceuticals.
© 2006 Janssen Pharmaceutical Companies
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 05/2019 PATIENT INFORMATION PREZISTA® (pre-ZIS-ta)
(darunavir)
oral suspensionPREZISTA® (pre-ZIS-ta)
(darunavir)
tabletRead this Patient Information before you start taking PREZISTA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Also read the Patient Information leaflet for ritonavir.What is the most important information I should know about PREZISTA? - Ask your healthcare provider or pharmacist about medicines that should not be taken with PREZISTA. For more information, see "Who should not take PREZISTA?" and "What should I tell my healthcare provider before taking PREZISTA?"
- PREZISTA may cause liver problems. Some people taking PREZISTA in combination with ritonavir have developed liver problems, which may be life-threatening. Your healthcare provider should do blood tests before and during your PREZISTA and ritonavir combination treatment. If you have chronic hepatitis B or C infection, your healthcare provider should check your blood tests more often because you have an increased chance of developing liver problems. Tell your healthcare provider if you have any of the below signs and symptoms of liver problems.
- dark (tea colored) urine
- yellowing of your skin or whites of your eyes
- pale colored stools (bowel movements)
- nausea
- vomiting
- pain or tenderness on your right side below your ribs
- loss of appetite
- tiredness
- PREZISTA may cause severe or life-threatening skin reactions or rash. Sometimes these skin reactions and skin rashes can become severe and require treatment in a hospital. Tell your healthcare provider right away if you develop a rash. Stop taking PREZISTA and ritonavir combination treatment and tell your healthcare provider right away if you have any skin changes with symptoms below:
- fever
- tiredness
- muscle or joint pain
- blisters or skin lesions
- mouth sores or ulcers
- red or inflamed eyes, like "pink eye" (conjunctivitis)
Rash occurred more often in people taking PREZISTA and raltegravir together than with either drug separately, but was generally mild.
See "What are the possible side effects of PREZISTA?" for more information about side effects.What is PREZISTA?
PREZISTA is a prescription HIV-1 (Human Immunodeficiency Virus-type 1) medicine used with ritonavir and other antiretroviral medicines to treat HIV-1 infection in adults and children 3 years of age and older. HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
PREZISTA should not be used in children under 3 years of age.
When used with other antiretroviral medicines to treat HIV-1 infection, PREZISTA may help:- reduce the amount of HIV-1 in your blood. This is called "viral load".
- increase the number of CD4+ (T) cells in your blood that help fight off other infections.
Reducing the amount of HIV-1 and increasing the CD4+ (T) cells in your blood may improve your immune system. This may reduce your risk of death or getting infections that can happen when your immune system is weak (opportunistic infections).
PREZISTA does not cure HIV-1 infection or AIDS. You must keep taking HIV-1 medicines to control HIV-1 infection and decrease HIV-related illnesses.
Avoid doing things that can spread HIV-1 infection to others:- Do not share or re-use needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
Ask your healthcare provider if you have any questions on how to prevent passing HIV to other people.
Who should not take PREZISTA?
Do not take PREZISTA with any medicine that contains:- alfuzosin
- cisapride
- colchicine, if you have liver or kidney problems
- dronedarone
- elbasvir and grazoprevir
- ergot-containing medicines:
- dihydroergotamine
- ergotamine tartrate
- methylergonovine
- ivabradine
- lomitapide
- lovastatin
- lurasidone
- midazolam, when taken by mouth
- naloxegol
- pimozide
- ranolazine
- rifampin
- sildenafil, when used for the treatment of pulmonary arterial hypertension (PAH)
- simvastatin
- St. John's wort (Hypericum perforatum)
- triazolam
Serious problems can happen if you or your child take any of these medicines with PREZISTA.
What should I tell my healthcare provider before taking PREZISTA?
Before taking PREZISTA, tell your healthcare provider if you:- have liver problems, including hepatitis B or hepatitis C
- are allergic to sulfa medicines
- have high blood sugar (diabetes)
- have hemophilia
- have any other medical conditions
- are pregnant or plan to become pregnant. Tell your healthcare provider if you become pregnant while taking PREZISTA.
- Pregnancy Registry: There is a pregnancy registry for women who take antiretroviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take PREZISTA.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if PREZISTA can pass into your breast milk.
- Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with PREZISTA. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with PREZISTA.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take PREZISTA with other medicines.
How should I take PREZISTA? - Take PREZISTA exactly as your healthcare provider tells you.
- You must take ritonavir at the same time as PREZISTA.
- Do not change your dose or stop treatment with PREZISTA without talking to your healthcare provider.
- Take PREZISTA and ritonavir with food.
- If you have difficulty swallowing PREZISTA tablets, PREZISTA oral suspension is also available. Your healthcare provider will help decide whether PREZISTA tablets or oral suspension is right for you.
- If your child is taking PREZISTA, your child's healthcare provider will decide the right dose based on your child's weight. Your child's healthcare provider will tell you how much PREZISTA (tablets or oral suspension) and how much ritonavir (capsules, tablets or solution) your child should take. Your child should take PREZISTA with ritonavir with food. If your child does not tolerate ritonavir oral solution, ask your child's healthcare provider for advice.
- PREZISTA oral suspension should be given with the supplied oral dosing syringe. Shake the suspension well before each use. See the "Instructions for Use" that come with PREZISTA oral suspension for information about the right way to prepare and take a dose.
- It is important that you do not miss or skip doses of PREZISTA during treatment.
- If you take too much PREZISTA, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of PREZISTA?
PREZISTA may cause serious side effects, including:- See "What is the most important information I should know about PREZISTA?"
- Diabetes and high blood sugar (hyperglycemia). Some people who take protease inhibitors including PREZISTA can get high blood sugar, develop diabetes, or your diabetes can get worse. Tell your healthcare provider if you notice an increase in thirst or urinate often while taking PREZISTA.
- Changes in body fat can happen in people who take HIV-1 medicines. The changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the middle of your body (trunk). Loss of fat from the legs, arms, and face may also happen. The exact cause and long-term health effects of these conditions are not known.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after starting your HIV-1 medicine.
- Increased bleeding for hemophiliacs. Some people with hemophilia have increased bleeding with protease inhibitors including PREZISTA.
The most common side effects of PREZISTA include:
- diarrhea
- nausea
- rash
- headache
- stomach-area (abdominal) pain
- vomiting
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of PREZISTA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store PREZISTA? - Store PREZISTA oral suspension and tablets at room temperature 77°F (25°C).
- Do not refrigerate or freeze PREZISTA oral suspension.
- Keep PREZISTA oral suspension away from high heat.
- PREZISTA oral suspension should be stored in the original container.
Keep PREZISTA and all medicines out of the reach of children.
General information about the safe and effective use of PREZISTA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PREZISTA for a condition for which it was not prescribed. Do not give PREZISTA to other people even if they have the same condition you have. It may harm them.
This leaflet summarizes the most important information about PREZISTA. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about PREZISTA that is written for health professionals. For more information, call 1-800-526-7736.What are the ingredients in PREZISTA?
Active ingredient: darunavir
Inactive ingredients:
PREZISTA oral suspension: citric acid monohydrate, hydrochloric acid (for pH adjustment), hydroxypropyl cellulose, masking flavor, methylparaben sodium, microcrystalline cellulose, purified water, sodium carboxymethylcellulose, strawberry cream flavor, and sucralose.
PREZISTA 75 mg and 150 mg tablets: colloidal silicon dioxide, crospovidone, magnesium stearate, microcrystalline cellulose. The film coating contains: OPADRY® White (polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
PREZISTA 600 mg tablets: colloidal silicon dioxide, crospovidone, magnesium stearate, microcrystalline cellulose. The film coating contains: OPADRY® Orange (FD&C Yellow No. 6, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
PREZISTA 800 mg tablets: colloidal silicon dioxide, crospovidone, magnesium stearate, microcrystalline cellulose, hypromellose. The film coating contains: OPADRY® Dark Red (iron oxide red, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
Product of Ireland
Manufactured by: PREZISTA oral suspension, Janssen Pharmaceutica NV, Beerse, Belgium
PREZISTA tablets, Janssen Ortho LLC, Gurabo, PR 00778 or Janssen Cilag SpA, Latina, IT
Manufactured for: Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560
PREZISTA® is a registered trademark of Janssen Pharmaceuticals
© 2006 Janssen Pharmaceutical Companies -
INSTRUCTIONS FOR USEPREZISTA (pre-ZIS-ta)(darunavir)oral suspension
Be sure that you read, understand, and follow these Instructions for Use so that you measure and take PREZISTA oral suspension correctly. Ask your healthcare provider if you are not sure.
Each PREZISTA oral suspension carton contains:
- 1 bottle of PREZISTA oral suspension
- Oral dosing syringe
- 1 oral syringe adapter
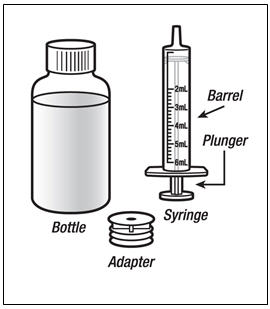
Important information for use:
- Shake PREZISTA oral suspension well before each use.
- PREZISTA oral suspension should be given with the oral dosing syringe provided to make sure you measure the right amount. The oral dosing syringe provided with your PREZISTA oral suspension should not be used for any other medicines.
- 1.
Shake the bottle.
- Shake the bottle well before each use (See Figure A).
- 2.
Open the bottle of PREZISTA oral suspension.
- Open the bottle by pushing downward on the cap and twisting it in the direction of the arrow (counter-clockwise) (See Figure B).
- 3.
The first time a bottle of PREZISTA oral suspension is used:
- Insert the oral syringe adapter into the bottle. Press on the adapter until it is even with the top of the bottle (See Figure C).
- Do not remove the oral syringe adapter from the bottle once inserted.
- 4.
Insert the oral dosing syringe.
- Fully push down (depress) the plunger of the syringe.
- Insert the syringe into the opening of the oral syringe adapter until it is firmly in place (See Figure D).
- 5.
Withdraw the prescribed amount of PREZISTA oral suspension.
- Turn the bottle upside down. Gently pull back the plunger of the syringe until the bottom of the plunger is even with the markings on the syringe for the prescribed dose (See Figure E). If you see air bubbles in the syringe, push the plunger in to empty the oral suspension back into the bottle. Then repeat steps 4 and 5.
- If you or your child's dose of PREZISTA oral suspension is more than 6 mL, you will need to divide the dose. Follow the instructions given to you by your healthcare provider or pharmacist about how to divide the dose.
- 6. Turn the bottle upright and remove the syringe from the bottle by pulling straight up on the oral dosing syringe (See Figure F).
- 7.
Take the dose of PREZISTA.
- Place the tip of the oral dosing syringe in the mouth.
- Press on the plunger of the syringe towards the mouth (See Figure G).
If you or your child's dose is more than 6 mL you will need to divide the dose. Follow the instructions given to you by your healthcare provider or pharmacist about how to divide the dose, and repeat steps 4 through 7.
- 8.
Close the bottle with the cap after use.
- Close the bottle by twisting the cap in the direction of the arrow (clockwise) (See Figure H).
- 9. Remove the plunger from the barrel by pulling the plunger and barrel away from each other (See Figure I). Rinse both parts of the syringe with water and allow to air dry after each use.
- 10. After air drying, put the oral dosing syringe back together by inserting the plunger into the barrel (See Figure J). Store the oral dosing syringe with PREZISTA oral suspension.
How should I store PREZISTA?
- Store PREZISTA oral suspension and the oral dosing syringe at room temperature 77°F (25°C).
- Do not refrigerate or freeze PREZISTA oral suspension.
- Keep PREZISTA oral suspension away from high heat.
- Store PREZISTA oral suspension in the original container.
Keep PREZISTA and all medicines out of the reach of children.
This Instruction for Use has been approved by the U.S. Food and Drug Administration.
Product of Ireland
Manufactured by:
Janssen Pharmaceutica, NV
Beerse, BelgiumManufactured for:
Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560Revised: January 2018
© 2006 Janssen Pharmaceutical Companies -
PRINCIPAL DISPLAY PANEL - 75 mg Tablet Bottle Label
480 Tablets
Rx onlyNDC: 59676-563-01
PREZISTA®
(darunavir) tablets75 mg
Each tablet contains darunavir
ethanolate equivalent to 75 mg
of darunavir.ALERT
Find out about
medicines that
should NOT be taken
with PREZISTA.janssen
-
PRINCIPAL DISPLAY PANEL - 150 mg Tablet Bottle Label
240 Tablets
Rx onlyNDC: 59676-564-01
PREZISTA®
(darunavir) tablets150 mg
Each tablet contains darunavir
ethanolate equivalent to 150 mg
of darunavir.ALERT
Find out about
medicines that
should NOT be taken
with PREZISTA.janssen
-
PRINCIPAL DISPLAY PANEL - 600 mg Tablet Bottle Label
60 Tablets
Rx onlyNDC: 59676-562-01
PREZISTA®
(darunavir) tablets600 mg
Each tablet contains darunavir
ethanolate equivalent to 600 mg
of darunavir.ALERT
Find out about
medicines that
should NOT be taken
with PREZISTA.janssen
-
PRINCIPAL DISPLAY PANEL - 800 mg Tablet Bottle Label
30 Tablets
Rx onlyNDC: 59676-566-30
PREZISTA®
(darunavir) tablets800 mg
Each tablet contains darunavir ethanolate
equivalent to 800 mg of darunavir.ALERT
Find out about medicines that
should NOT be taken with
PREZISTA.janssen

-
PRINCIPAL DISPLAY PANEL - 200 mL Bottle Carton
200 mL
NDC: 59676-565-01PREZISTA®
(darunavir) Oral Suspension100 mg per mL
Store at 25°C (77°F); with excursions
permitted to 15°-30°C (59°-86°F).Do not refrigerate or freeze.
Shake well before each usage.
Dosage: See package insert.
Dispensing: For information concerning
proper usage of the oral dosing syringe,
see accompanying patient instructions.Keep out of reach of children.
ALERT
Find out about medicines that
should NOT be taken with
PREZISTA.Rx only
janssen
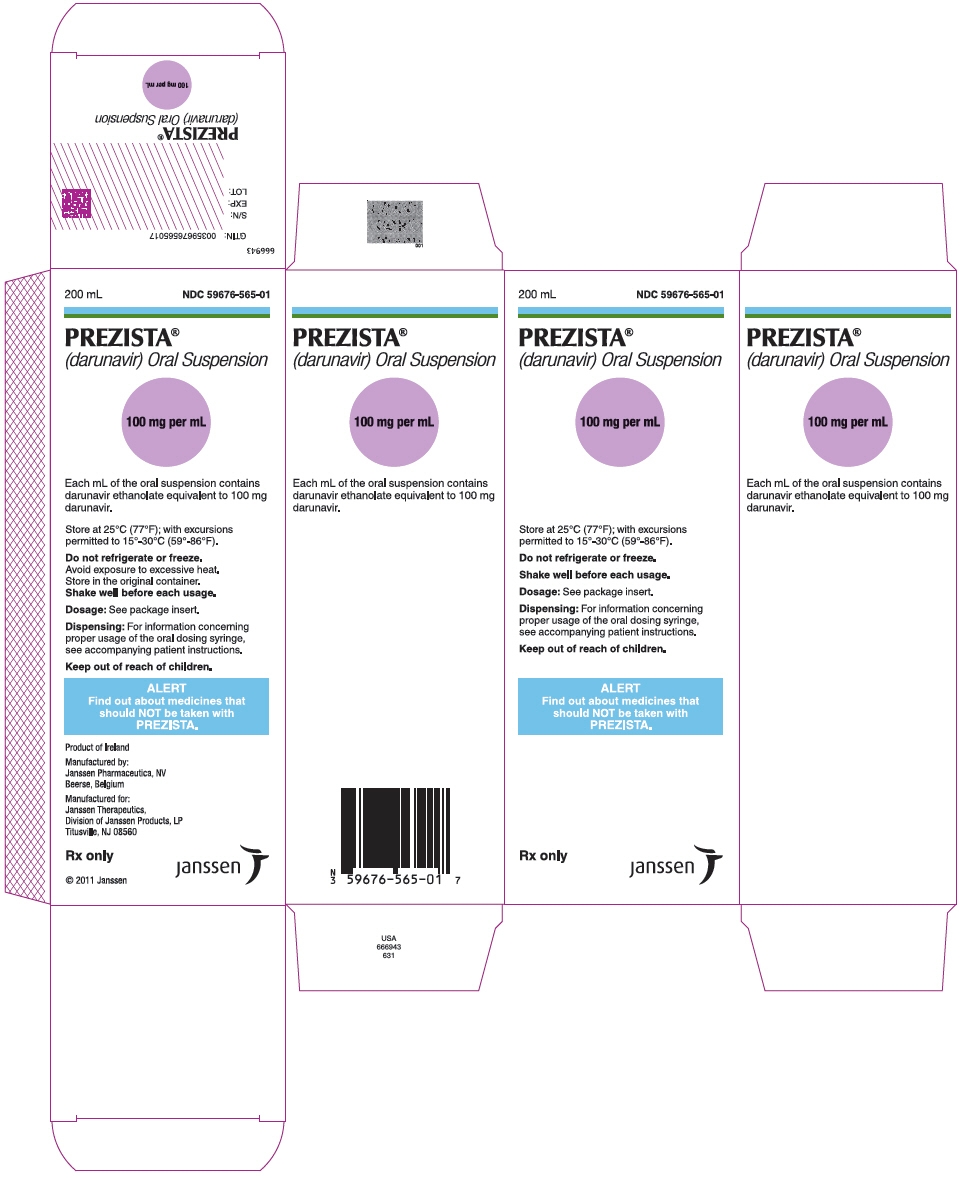
-
INGREDIENTS AND APPEARANCE
PREZISTA
darunavir tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59676-563 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength darunavir ethanolate (UNII: 33O78XF0BW) (darunavir - UNII:YO603Y8113) darunavir 75 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) crospovidone (15 mpa.s at 5%) (UNII: 68401960MK) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) polyethylene glycol 3350 (UNII: G2M7P15E5P) polyvinyl alcohol, unspecified (UNII: 532B59J990) talc (UNII: 7SEV7J4R1U) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL (caplet-shaped) Size 9mm Flavor Imprint Code 75;TMC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59676-563-01 480 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/14/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021976 01/14/2009 PREZISTA
darunavir tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59676-564 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength darunavir ethanolate (UNII: 33O78XF0BW) (darunavir - UNII:YO603Y8113) darunavir 150 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) crospovidone (15 mpa.s at 5%) (UNII: 68401960MK) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) polyethylene glycol 3350 (UNII: G2M7P15E5P) polyvinyl alcohol, unspecified (UNII: 532B59J990) talc (UNII: 7SEV7J4R1U) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL (oval-shaped) Size 14mm Flavor Imprint Code 150;TMC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59676-564-01 240 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/27/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021976 04/27/2009 PREZISTA
darunavir tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59676-562 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength darunavir ethanolate (UNII: 33O78XF0BW) (darunavir - UNII:YO603Y8113) darunavir 600 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) crospovidone (15 mpa.s at 5%) (UNII: 68401960MK) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) FD&C Yellow No. 6 (UNII: H77VEI93A8) polyethylene glycol 3350 (UNII: G2M7P15E5P) polyvinyl alcohol, unspecified (UNII: 532B59J990) talc (UNII: 7SEV7J4R1U) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE Score no score Shape OVAL (oval-shaped) Size 21mm Flavor Imprint Code 600MG;TMC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59676-562-01 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/08/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021976 03/08/2008 PREZISTA
darunavir tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59676-566 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength darunavir ethanolate (UNII: 33O78XF0BW) (darunavir - UNII:YO603Y8113) darunavir 800 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) crospovidone (15 mpa.s at 5%) (UNII: 68401960MK) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) hypromellose, unspecified (UNII: 3NXW29V3WO) polyethylene glycol 3350 (UNII: G2M7P15E5P) polyvinyl alcohol, unspecified (UNII: 532B59J990) talc (UNII: 7SEV7J4R1U) titanium dioxide (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color RED (dark red) Score no score Shape OVAL (oval-shaped) Size 20mm Flavor Imprint Code 800;T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59676-566-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/09/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021976 11/09/2012 PREZISTA
darunavir suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59676-565 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength darunavir ethanolate (UNII: 33O78XF0BW) (darunavir - UNII:YO603Y8113) darunavir 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) microcrystalline cellulose (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) METHYLPARABEN SODIUM (UNII: CR6K9C2NHK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SUCRALOSE (UNII: 96K6UQ3ZD4) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) STRAWBERRY (UNII: 4J2TY8Y81V) Product Characteristics Color WHITE (white to off-white opaque) Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59676-565-01 1 in 1 CARTON 12/16/2011 1 200 mL in 1 BOTTLE, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202895 12/16/2011 Labeler - Janssen Products LP (804684207) Establishment Name Address ID/FEI Business Operations JANSSEN ORTHO LLC 805887986 ANALYSIS(59676-563, 59676-564, 59676-562, 59676-565, 59676-566) , MANUFACTURE(59676-563, 59676-564, 59676-562, 59676-566) Establishment Name Address ID/FEI Business Operations Cilag AG 483237103 API MANUFACTURE(59676-563, 59676-564, 59676-562, 59676-565, 59676-566) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutical Sciences Unlimited Company 985639841 API MANUFACTURE(59676-563, 59676-564, 59676-562, 59676-565, 59676-566) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceuticals, Inc 063137772 ANALYSIS(59676-563, 59676-564, 59676-562, 59676-565, 59676-566) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutica NV 370005019 MANUFACTURE(59676-565) , ANALYSIS(59676-565) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutica NV 374747970 API MANUFACTURE(59676-565) Establishment Name Address ID/FEI Business Operations Janssen Cilag SpA 542797928 MANUFACTURE(59676-562, 59676-566, 59676-563, 59676-564) , ANALYSIS(59676-562, 59676-566, 59676-563, 59676-564)
Trademark Results [PREZISTA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PREZISTA 78762650 3236346 Live/Registered |
JANSSEN SCIENCES IRELAND UNLIMITED COMPANY 2005-11-29 |
 PREZISTA 77239629 not registered Dead/Abandoned |
Tibotec Pharmaceuticals Ltd. 2007-07-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.