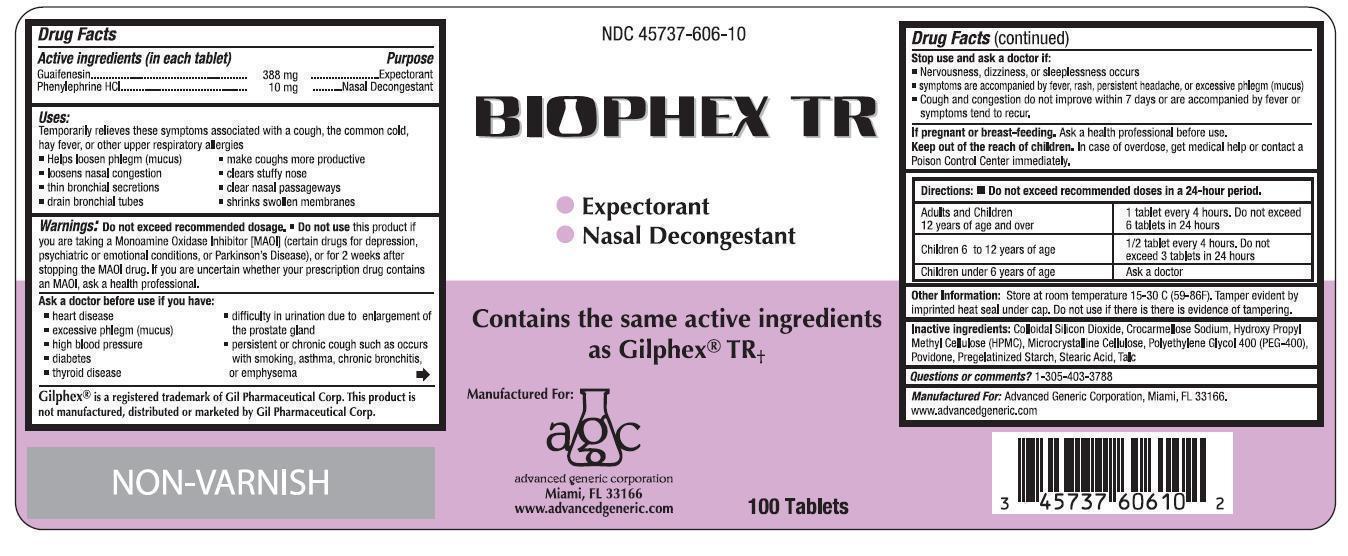
BIOPHEX TR- guaifenesin, phenylephrine hcl tablet
Biophex by
Drug Labeling and Warnings
Biophex by is a Otc medication manufactured, distributed, or labeled by Advanced Generic Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
Temporarily relieves these symptoms associated with a cough, the common cold, hay fever, or other upper respiratory allergies
- helps loosen phlegm (mucus)
- loosens nasal congestions
- thin bronchial secretions
- drain bronchial tubes
- make coughs more productive
- clears stuffy nose
- clear nasal passageways
- shrinks swollen membranes
-
WARNINGS
Warnings
Do not exceed recommended dosage
Do not use this product if you are taking a Monoamine Oxidase Inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's Disease), or for 2 weeks after stopping the MAOI drug. If you are uncertain whether your prescription drug contains an MAOI, ask a health professional.
Ask a doctor before use if you have:
- heart disease
- excessive phlegm (mucus)
- high blood pressure
- diabetes
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions: Do not exceed recommended doses in a 24-hour period
Adults and children 12 years of age and over 1 tablet every 4 hours. Do not exceed 6 tablets in 24 hours
Children 6 to 12 years of age 1/2 tablet every 4 hours. Do not exceed 3 tablets in 24 hours
Children under 6 years of age Ask a doctor
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOPHEX TR
guaifenesin, phenylephrine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 45737-606 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 388 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE (UNII: FZ989GH94E) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white Score no score Shape OVAL Size 18mm Flavor Imprint Code BP;AGC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45737-606-10 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 04/01/2014 Labeler - Advanced Generic Corporation (831762971)
Trademark Results [Biophex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIOPHEX 76547143 2881166 Dead/Cancelled |
Reed Elsevier Properties Inc. 2003-09-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.