Baclofen by Sarras Health LLC BACLOFEN solution
Baclofen by
Drug Labeling and Warnings
Baclofen by is a Prescription medication manufactured, distributed, or labeled by Sarras Health LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use baclofen oral solution safely and effectively. See full prescribing information for baclofen oral solution.
BACLOFEN oral solution
Initial U.S. Approval: 1977INDICATIONS AND USAGE
- Baclofen oral solution is a gamma-aminobutyric acid (GABA-ergic) agonist indicated for the treatment of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity. ( 1)
- Baclofen oral solution may also be of some value in patients with spinal cord injuries and other spinal cord diseases. ( 1)
Limitations of Use
Baclofen oral solution is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders ( 1)
DOSAGE AND ADMINISTRATION
- Baclofen oral solution is available in multiple concentrations; include both the total dose in mg and the total dose in volume on prescriptions ( 2.1)
- Initiate baclofen oral solution with a low dosage, preferably in divided doses, administered orally. Increase gradually based on clinical response and tolerability. ( 2.2)
- The maximum dosage is 80 mg daily (20 mg four times a day). ( 2.2)
- When discontinuing, reduce the dosage slowly. ( 2.3)
DOSAGE FORMS AND STRENGTHS
Oral Solution: 10 mg/5 mL ( 3)
CONTRAINDICATIONS
- Hypersensitivity to baclofen ( 4)
WARNINGS AND PRECAUTIONS
- Abrupt discontinuation of baclofen has resulted in serious adverse reactions including death; therefore, reduce the dosage slowly when baclofen oral solution is discontinued. ( 5.1)
- Neonatal withdrawal symptoms can occur; gradually reduce the dosage and discontinue baclofen oral solution before delivery. ( 5.2)
- Baclofen oral solution can cause drowsiness and sedation. Patients should avoid the operation of automobiles or other dangerous machinery until they know how the drug affects them. Advise patients that the central nervous system effects of baclofen oral solution may be additive to those of alcohol and other CNS depressants. ( 5.3)
- Baclofen oral solution can cause exacerbation of the following: psychotic disorders, schizophrenia, or confusional states; autonomic dysreflexia; epilepsy. Use with caution in patients with these conditions ( 5.5, 5.6, 5.7)
ADVERSE REACTIONS
- The most common (up to 15% or more) adverse reactions in patients were drowsiness, dizziness, and weakness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sarras Health LLC at 1-888-570-5004 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
2.2 Recommended Dosage
2.3 Discontinuation of Baclofen Oral Solution
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Adverse Reactions from Abrupt Withdrawal of Baclofen Oral Solution
5.2 Neonatal Withdrawal Symptoms
5.3 Drowsiness and Sedation
5.4 Poor Tolerability in Stroke Patients
5.5 Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional States
5.6 Exacerbation of Autonomic Dysreflexia
5.7 Exacerbation of Epilepsy
5.8 Posture and Balance Effects
5.9 Ovarian Cysts
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 CNS Depressants and Alcohol
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
10.1 Symptoms of Baclofen Overdose
10.2 Treatment for Overdose
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Baclofen oral solution is indicated for the treatment of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity.
Baclofen oral solution may also be of some value in patients with spinal cord injuries and other spinal cord diseases.
Limitations of Use
Baclofen oral solution is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders. -
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
Baclofen oral solution is available in multiple concentrations; ensure accuracy when prescribing, dispensing and administering baclofen oral solution to avoid dosing errors due to confusion between mg and mL, and with other baclofen oral solutions of different concentrations. When writing prescriptions, include both the total dose in mg and the total dose in volume.
2.2 Recommended Dosage
Initiate baclofen oral solution with a low dosage, preferably in divided doses, administered orally. The following gradually increasing dosage regimen is suggested, but should be adjusted based on clinical response and tolerability:
5 mg (2.5 mL) three times a day for three days
10 mg (5 mL) three times a day for three days
15 mg (7.5 mL) three times a day for three days
20 mg (10 mL) three times a day for three days
Additional increases may be necessary up to the maximum recommended dosage of 80 mg daily (20 mg four times a day).
2.3 Discontinuation of Baclofen Oral Solution
When discontinuing baclofen oral solution, reduce the dosage slowly and avoid abrupt withdrawn from the drug to help minimize the risk of adverse reactions [see Warnings and Precautions ( 5.1)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Adverse Reactions from Abrupt Withdrawal of Baclofen Oral Solution
Abrupt discontinuation of baclofen, regardless of the cause, has resulted in adverse reactions that include hallucinations, seizures, high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity, that in rare cases has advanced to rhabdomyolysis, multiple organ-system failure, and death. Therefore, reduce the dosage slowly when baclofen oral solution is discontinued, unless the clinical situation justifies a rapid withdrawal.
5.2 Neonatal Withdrawal Symptoms
Withdrawal symptoms in neonates whose mothers were treated with oral baclofen throughout pregnancy have been reported starting hours to days after delivery. The symptoms of withdrawal in these infants have included increased muscle tone, tremor, jitteriness, and seizure. If the potential benefit justifies the potential risk to the fetus and baclofen oral solution is continued during pregnancy, gradually reduce the dosage and discontinue baclofen oral solution before delivery. If slow withdrawal is not feasible, advise the parents or caregivers of the exposed neonate of the potential for neonatal withdrawal.
5.3 Drowsiness and Sedation
Drowsiness and sedation have been reported in up to 63% of patients taking baclofen, the active ingredient in baclofen oral solution [see Adverse Reactions ( 6.1)] . Patients should avoid operation of automobiles or other dangerous machinery and activities made hazardous by decreased alertness when starting baclofen oral solution or increasing the dose until they know how the drug affects them. Advise patients that the central nervous system depressant effects of baclofen oral solution may be additive to those of alcohol and other CNS depressants.
5.4 Poor Tolerability in Stroke Patients
Baclofen oral solution should be used with caution in patients who have had a stroke. Baclofen has not significantly benefited patients with stroke. These patients have also shown poor tolerability to the drug.
5.5 Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional States
Baclofen oral solution should be used with caution in patients suffering from psychotic disorders, schizophrenia, or confusional states. If treated with baclofen oral solution, these patients should be kept under careful surveillance because exacerbations of these conditions have been observed with oral baclofen administration.
5.6 Exacerbation of Autonomic Dysreflexia
Baclofen oral solution should be used with caution in patients with a history of autonomic dysreflexia. The presence of nociceptive stimuli or abrupt withdrawal of baclofen oral solution may cause an autonomic dysreflexic episode.
5.7 Exacerbation of Epilepsy
Baclofen oral solution should be used with caution in patients with epilepsy. Deterioration in seizure control has been reported in patients taking baclofen.
5.8 Posture and Balance Effects
Baclofen oral solution should be used with caution in patients where spasticity is utilized to sustain upright posture and balance in locomotion or whenever spasticity is utilized to obtain increased function.
5.9 Ovarian Cysts
A dose-related increase in incidence of ovarian cysts was observed in female rats treated chronically with oral baclofen. Ovarian cysts have been found by palpation in about 4% of the multiple sclerosis patients who were treated with oral baclofen for up to one year. In most cases, these cysts disappeared spontaneously while patients continued to receive the drug. Ovarian cysts are estimated to occur spontaneously in approximately 1% to 5% of the normal female population.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Adverse Reactions from Abrupt Withdrawal of Baclofen Oral Solution [see Warnings and Precautions ( 5.1)]
- Neonatal Withdrawal Symptoms [see Warnings and Precautions ( 5.2)]
- Drowsiness and Sedation [see Warnings and Precautions ( 5.3)]
- Poor Tolerability in Stroke Patients [see Warnings and Precautions ( 5.4)]
- Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional States [see Warnings and Precautions ( 5.5)]
- Exacerbation of Autonomic Dysreflexia [see Warnings and Precautions ( 5.6)]
- Exacerbation of Epilepsy [see Warnings and Precautions ( 5.7)]
- Posture and Balance Effects [see Warnings and Precautions ( 5.8)]
- Ovarian Cysts [see Warnings and Precautions ( 5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reaction is transient drowsiness. In one controlled study of 175 patients, transient drowsiness was observed in 63% of those receiving baclofen compared to 36% of those in the placebo group. Other common adverse reactions (up to 15%) are dizziness and weakness. Adverse reactions with a frequency of ≥1% are listed in Table 1.
Table 1. Common (≥1%) Adverse Reactions in Patients Treated with Baclofen for Spasticity ADVERSE REACTION
PERCENT
Drowsiness
10-63%
Dizziness
5-15%
Weakness
5-15%
Nausea
4-12%
Confusion
1-11%
Hypotension
0-9%
Headache
4-8%
Insomnia
2-7%
Constipation
2-6%
Urinary Frequency
2-6%
Fatigue
2-4%
The following adverse reactions not included in Table 1, classified by body system, were also reported:
Neuropsychiatric:euphoria, excitement, depression, hallucinations, paresthesia, muscle pain, tinnitus, slurred speech, coordination disorder, tremor, rigidity, dystonia, ataxia, blurred vision, nystagmus, strabismus, miosis, mydriasis, diplopia, dysarthria, epileptic seizure
Cardiovascular:dyspnea, palpitation, chest pain, syncope
Gastrointestinal:dry mouth, anorexia, taste disorder, abdominal pain, vomiting, diarrhea, and positive test for occult blood in stool
Genitourinary:enuresis, urinary retention, dysuria, impotence, inability to ejaculate, nocturia, hematuria
Other:rash, pruritus, ankle edema, excessive perspiration, weight gain, nasal congestion
The following laboratory tests have been found to be abnormal in patients receiving baclofen: increased SGOT, elevated alkaline phosphatase, and elevation of blood sugar.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of baclofen oral solution in pregnant women. Oral administration of baclofen to pregnant rats resulted in an increased incidence of fetal structural abnormalities at a dose which was also associated with maternal toxicity. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.Clinical Considerations
Fetal/Neonatal adverse reactions
Baclofen oral solution may increase the risk of late-onset neonatal withdrawal symptoms [see Warnings and Precautions ( 5.2)] .Data
Animal Data
Baclofen given orally has been shown to increase the incidence of omphaloceles (ventral hernias) in fetuses of rats given approximately 13 times on a mg/kg basis, or 3 times on a mg/m 2basis, the maximum oral dose recommended for human use; this dose also caused reductions in food intake and weight gain in the dams. This abnormality was not seen in mice or rabbits.8.2 Lactation
Risk Summary
At recommended oral doses, baclofen is present in human milk. There are no human data on the effects of baclofen on milk production. There are no adequate data on the effects of baclofen on the breastfed infant. Withdrawal symptoms can occur in breastfed infants when maternal administration of baclofen oral solution is stopped, or when breastfeeding is stopped [see Warnings and Precautions ( 5.2)] .The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for baclofen oral solution and any potential adverse effects on the breastfed infant from baclofen oral solution or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 have not been established.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Baclofen oral solution is a gamma-aminobutyric acid (GABA-ergic) agonist available as 10 mg/5 mL solution for oral administration. Its chemical name is 4-amino-3-(4-chlorophenyl)-butanoic acid, and its structural formula is:
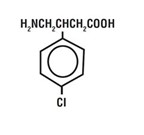
Molecular formula is C 1OH 12CINO 2.
Molecular Weight is 213.66.
Baclofen USP is a white to off-white, odorless or practically odorless crystalline powder. It is slightly soluble in water, very slightly soluble in methanol, and insoluble in chloroform.
The baclofen oral solution inactive ingredients are: glycerin, methylparaben, propylparaben, purified water, and sucralose. May also contain sodium hydroxide or hydrochloric acid for pH adjustment.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism of action of baclofen is not fully understood. Baclofen inhibits both monosynaptic and polysynaptic reflexes at the spinal level, possibly by decreasing excitatory neurotransmitter release from afferent terminals, although actions at supraspinal sites may also occur and contribute to its clinical effect. Baclofen is a structural analog of the inhibitory neurotransmitter gamma- aminobutyric acid (GABA), and may exert its effects by stimulation of the GABA Breceptor subtype.
12.2 Pharmacodynamics
Baclofen has been shown to have general CNS depressant properties, as indicated by the production of sedation with tolerance, somnolence, ataxia, and respiratory and cardiovascular depression [see Warnings and Precautions ( 5.3), Adverse Reactions ( 6.1), and Overdosage ( 10.1)].
12.3 Pharmacokinetics
A pharmacokinetic study in heathy adult male subjects under fasting conditions at 20 mg dose level demonstrated similar bioavailability for baclofen oral solution (5 mg/5 mL) and oral tablets. The peak plasma concentrations were achieved in about 0.75 hours from oral solution and the apparent elimination half-life is about 5.7 hours. Baclofen is excreted primarily by the kidney in unchanged form, and there is relatively large intersubject variation in absorption and/or elimination.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No increase in tumors was seen in rats receiving baclofen orally for two years at approximately 30 to 60 times on a mg/kg basis, or 10 to 20 times on a mg/ m 2basis, the maximum oral dose recommended for human use.
Mutagenesis
Genetic toxicology assays have not been conducted for baclofen.
Impairment of Fertility
Studies to evaluate the effects of baclofen on fertility have not been conducted.
-
14 CLINICAL STUDIES
The efficacy of baclofen oral solution is based upon a bioavailability study in healthy adults comparing baclofen oral tablets to baclofen oral solution [see Clinical Pharmacology ( 12.3)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
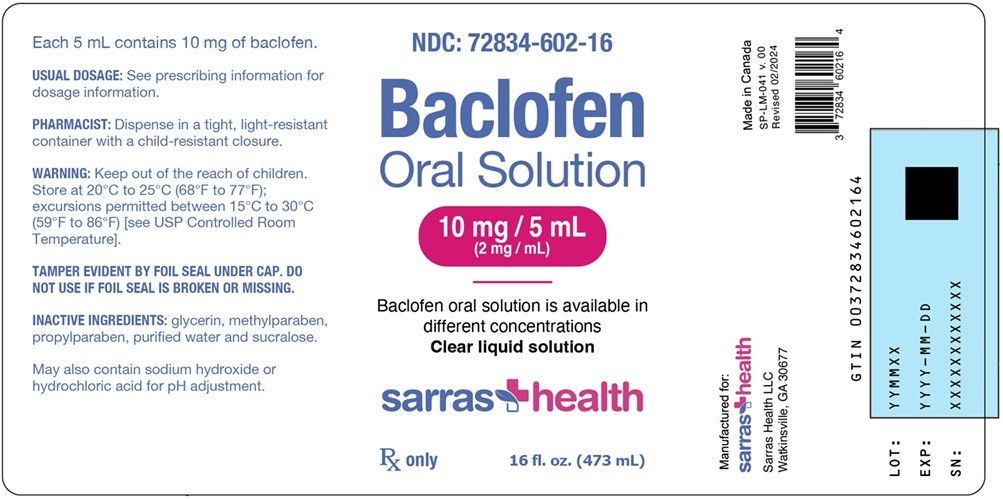
Baclofen Oral Solution contains 10 mg/5 mL baclofen. It is a clear, colorless solution and is supplied in bottles of 473 mL (NDC: 72834-602-16).
-
17 PATIENT COUNSELING INFORMATION
Administration Instructions
Instruct patients or caregivers to use an oral dosing syringe to correctly measure the prescribed amount of medication. Inform patients that oral dosing syringes may be obtained from their pharmacy.
Risks Related to Sudden Withdrawal of Baclofen Oral Solution
Advise patients and caregivers not to discontinue use of baclofen oral solution without consulting with their healthcare provider because sudden withdrawal of baclofen oral solution can result in serious complications that include hallucinations, seizures, high fever, confusion, muscle stiffness, multiple organ-system failure, and death [see Warnings and Precautions ( 5.1)]. Inform patients that early symptoms of baclofen oral solution withdrawal may include increased spasticity, itching, and tingling of extremities.
Neonatal Withdrawal Symptoms
Advise patients to notify their healthcare provider if they are pregnant, plan to become pregnant, or plan to breastfeed [see Warnings and Precautions ( 5.2) and Use in Specific Populations ( 8.2)].
Increased Risk of Drowsiness with Alcohol and Other CNS Depressants
Advise patients that baclofen oral solution may cause drowsiness, and that they should avoid the operation of automobiles or other dangerous machinery, or activities made hazardous by decreased alertness when starting baclofen oral solution or increasing the dose until they know how the drug affects them [see Warnings and Precautions ( 5.3)]. Inform patients and their caregivers that the drowsiness associated with baclofen oral solution use can be worsened by alcohol and other CNS depressants. Advise patients to read all medicine labels carefully, and to tell their healthcare provider about all prescription and nonprescription drugs they may use.
Distributed by:
Sarras Health, LLC
Watkinsville, GA 30677
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACLOFEN
baclofen solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72834-602 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACLOFEN (UNII: H789N3FKE8) (BACLOFEN - UNII:H789N3FKE8) BACLOFEN 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72834-602-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA208193 03/05/2024 Labeler - Sarras Health LLC (116763528)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.