LIDOCAINE HYDROCHLORIDE solution
Lidocaine Hydrochloride by
Drug Labeling and Warnings
Lidocaine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by A-S Medication Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Rx Only
-
BOXED WARNING
(What is this?)
WARNING: Life-threatening and fatal events in infants and young children
Postmarketing cases of seizures, cardiopulmonary arrest, and death in patients under the age of 3 years have been reported with use of Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% when it was not administered in strict adherence to the dosing and administration recommendations. In the setting of teething pain, Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% should generally not be used. For other conditions, the use of the product in patients less than 3 years of age should be limited to those situations where safer alternatives are not available or have been tried but failed.
To decrease the risk of serious adverse events with use of Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2%, instruct caregivers to strictly adhere to the prescribed dose and frequency of administration and store the prescription bottle safely out of reach of children.
-
DESCRIPTION
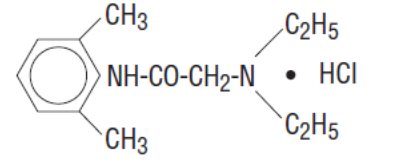
Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains a local anesthetic agent and is administered topically. Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains lidocaine hydrochloride, which is chemically designated as acetamide, 2-(diethylamino)-N- (2,6 dimethylphenyl)-, monohydrochloride and has the following structural formula:
The molecular formula of lidocaine is C14H22N2O. The molecular weight is 234.34.
-
COMPOSITION OF SOLUTION
Each mL contains 20 mg of lidocaine HCl. In addition each mL contains the following inactive ingredients: Carboxymethylcellulose sodium, methylparaben, natural orange flavor, propylparaben, purified water, and saccharin sodium. The pH is adjusted to 5.0 to 7.0 by means of hydrochloric acid and/or sodium hydroxide.
-
CLINICAL PHARMACOLOGY
Mechanism of Action:
Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Hemodynamics:
Excessive blood levels may cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. These changes may be attributable to a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system. The net effect is normally a modest hypotension when the recommended dosages are not exceeded.
Pharmacokinetics and Metabolism:
Lidocaine is absorbed following topical administration to mucous membranes, its rate and extent of absorption being dependent upon concentration and total dose administered, the specific site of application, and duration of exposure. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation in the liver. The plasma binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein.
Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline.
The elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to 2.0 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6.0 mcg free base per mL. In the rhesus monkey arterial blood levels of 18to21 mcg/mL have been shown to be threshold for convulsive activity.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
EXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS. PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ADMINISTRATION GUIDELINES AS SET FORTH IN THIS PACKAGE INSERT.
THE MANAGEMENT OF SERIOUS ADVERSE REACTIONS MAY REQUIRE THE USE OF RESUSCITATIVE EQUIPMENT, OXYGEN, AND OTHER RESUSCITATIVE DRUGS.
Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% should be used with extreme caution if the mucosa in the area of application has been traumatized, since under such conditions there is the potential for rapid systemic absorption.
Life-threatening and fatal events in infants and young children
Postmarketing cases of seizures, cardiopulmonary arrest, and death in patients under the age of 3 years have been reported with use of Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% when it was not administered in strict adherence to the dosing and administration recommendations. In the setting of teething pain, Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% should generally not be used. For other conditions, the use of the product in patients less than 3 years of age should be limited to those situations where safer alternatives are not available or have been tried but failed.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
-
PRECAUTIONS
Information for Patients:
Parents and caregivers should be cautioned about the following:
- For patients under 3 years of age, special care must be given to accurately measuring the prescribed dose and not administering the product more often than prescribed.
- To ensure accuracy, we recommend you use a measuring device to carefully measure the correct volume.
- The product should only be used for the prescribed indication.
- To reduce the risk of accidental ingestion, the product container should be tightly closed and the product should be stored well out of reach of all children immediately after each use.
- If the patient shows signs of systemic toxicity (e.g., lethargy, shallow breathing, seizure activity) emergency medical attention should be sought immediately and no additional product should be administered.
- Unused product should be discarded in a manner that prevents possible exposure to children and pets.
- All patients should be aware that when topical anesthetics are used in the mouth or throat, the production of topical anesthesia may impair swallowing and thus enhance the danger of aspiration. For this reason, food should not be ingested for 60 minutes following use of local anesthetic preparations in the mouth or throat area. This is particularly important in children because of their frequency of eating.
- Numbness of the tongue or buccal mucosa may increase the danger of biting trauma. For this reason food and/or chewing gum should not be used while the mouth or throat area is anesthetized.
- Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
General:
The safety and effectiveness of lidocaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies (See WARNINGS and ADVERSE REACTIONS). The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Repeated doses of lidocaine may cause significant increases in blood levels with each repeated dose because of slow accumulation of the drug and/or its metabolites. Tolerance varies with the status of the patient. Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age, weight and physical condition. Lidocaine should also be used with caution in patients with severe shock or heart block.
Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% should be used with caution in persons with known drug sensitivities. Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to lidocaine.
Drug Interactions:
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia:
Class
Examples
Nitrates/Nitrites
nitric oxide, nitroglycerin, nitroprusside, nitrous oxide
Local anesthetics
articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine
Antineoplastic agents
cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase
Antibiotics
dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides
Antimalarials
chloroquine, primaquine
Anticonvulsants
phenobarbital, phenytoin, sodium valproate
Other drugs
acetaminophen, metoclopramide, quinine, sulfasalazine
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Pregnancy:
Teratogenic Effects. Pregnancy Category B.
Reproduction studies have been performed in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine is administered to nursing women.
Pediatric Use:
Dosages in children should be reduced, commensurate with age, body weight and physical condition. (See DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy, or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central Nervous System:
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest.
Drowsiness following the administration of lidocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular System:
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic:
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent or to the methylparaben and/or propylparaben used in this formulation. Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
To report SUSPECTED ADVERSE REACTIONS, contact Hi-Tech Pharmacal Co., Inc. at 1-800-262-9010 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics. (See ADVERSE REACTIONS, WARNINGS, and PRECAUTIONS).
Management of Local Anesthetic Emergencies:
The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic administration.
The first step in the management of convulsions consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen. In situations where trained personnel are readily available, ventilation should be maintained and oxygen should be delivered by a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to use of local anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor as indicated by the clinical situation (e.g., ephedrine).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
Dialysis is of negligible value in the treatment of acute overdosage with lidocaine.
The oral LD50 of lidocaine in non-fasted female rats is 459 (346-773) mg/kg (as the salt) and 214 (159-324) mg/kg (as the salt) in fasted female rats.
-
DOSAGE AND ADMINISTRATION
Adult:
The maximum recommended single dose of Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% for healthy adults should be such that the dose of lidocaine HCI does not exceed 4.5 mg/kg or 2 mg/lb body weight and does not in any case exceed a total of 300 mg.
For symptomatic treatment of irritated or inflamed mucous membranes of the mouth and pharynx, the usual adult dose is 15 mL undiluted. For use in the mouth, the solution should be swished around in the mouth and spit out. For use in the pharynx, the undiluted solution should be gargled and may be swallowed. This dose should not be administered at intervals of less than three hours, and not more than eight doses should be given in a 24-hour period. The dosage should be adjusted commensurate with the patient's age, weight and physical condition. (See PRECAUTIONS).
Pediatric:
Care must be taken to ensure correct dosage in all pediatric patients as there have been cases of overdose due to inappropriate dosing.
It is difficult to recommend a maximum dose of any drug for children since this varies as a function of age and weight. For children over 3 years of age who have a normal lean body mass and normal body development, the maximum dose is determined by the child's weight or age. For example: in a child of 5 years weighing 50 lbs., the dose of lidocaine hydrochloride should not exceed 75-100 mg (3.7 to 5 mL of Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2%).
For infants and in children under 3 years of age, the solution should be accurately measured and no more than 1.2 mL be applied to the immediate area with a cotton-tipped applicator. Wait at least 3 hours before giving the next dose; a maximum of four doses may be given in a 12-hour period. Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2%) should only be used if the underlying condition requires treatment with a volume of product that is less than or equal to 1.2 mL.
- HOW SUPPLIED
- Lidocaine Hydrochloride
-
INGREDIENTS AND APPEARANCE
LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50090-0255(NDC:50383-775) Route of Administration ORAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE ANHYDROUS (UNII: EC2CNF7XFP) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) METHYLPARABEN (UNII: A2I8C7HI9T) ORANGE (UNII: 5EVU04N5QU) PROPYLPARABEN (UNII: Z8IX2SC1OH) SACCHARIN SODIUM (UNII: SB8ZUX40TY) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50090-0255-0 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/29/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040014 07/10/1995 Labeler - A-S Medication Solutions (830016429) Establishment Name Address ID/FEI Business Operations A-S Medication Solutions 830016429 RELABEL(50090-0255)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.