ACETAMINOPHEN ORAL SOLUTION solution DIPHENHYDRAMINE HYDROCHLORIDE liquid MILK OF MAGNESIA- magnesium hydroxide suspension
Milk of Magnesia by
Drug Labeling and Warnings
Milk of Magnesia by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals, Plastikon Healthcare, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Acetaminophen Oral Solution 160 mg/ 5 mL Unit Dose Cup
Major Pharmaceutical

 NDC: 0904-6738-70
NDC: 0904-6738-70
Acetaminophen
Oral Solution, USP
160 mg / 5 mL
Delivers 5 mL
See Insert
For Institutional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 48152
Sugar Free - Dye Free - Alcohol Free
Acetaminophen 160 mg / 5 mL Unit Dose Cup
Major PharmaceuticalsDirections
Do not use more than directed Shake well before use
Age (yr)
Dose (mL)
adults
- take 20 mL (640 mg) every 4 to 6 hours
- not to exceed 6 doses in a 24-hour period
- do not use more than 10 days unless directed by a doctor
under 18 years of age
- ask a doctor
Acetaminophen 160 mg / 5 mL
Major PharmaceuticalsKeep out of reach of children.
Overdose warning: taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Acetaminophen 160 mg / 5 mL
Major PharmaceuticalsDo not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any other inactive ingredients in this product
____________________________________________________________________________
Ask a doctor before use if the user
- has liver disease - is pregnant or breast-feeding ____________________________________________________________________________
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
____________________________________________________________________________
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days - new symptoms occur
- fever gets worse or lasts more than 3 days - redness or swelling is present
These could be signs of a serious condition
Acetaminophen 160 mg / 5 mL
Major PharmaceuticalsInactive ingredients cherry flavor, citric acid, glycerin, methylcellulose, microcrystalline cellulose, propyl paraben, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xantham gum
Acetaminophen 160 mg / 5 mL
Major PharmaceuticalsUses temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- muscular aches
- backache
- premenstrual and menstrual cramps
- the common cold
- headache
- toothache
- temporarily reduces fever
Acetaminophen 160 mg / 5 mL
Major PharmaceuticalsActive ingredient (in each 5 mL cup) Purpose Acetaminophen USP 160 mg…………………………..………………..Pain reliever / fever reducer
Acetaminophen 160 mg / 5 mL
Major PharmaceuticalsOther information
- store at 20°-25°C (68°-77°F). Avoid excessive heat 40°C (104°F)
- protect from excessive moisture - do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free - see bottom of cup for lot number and expiration date
-
Diphenhydramine HCl 12.5 mg/ 5 mL Cups

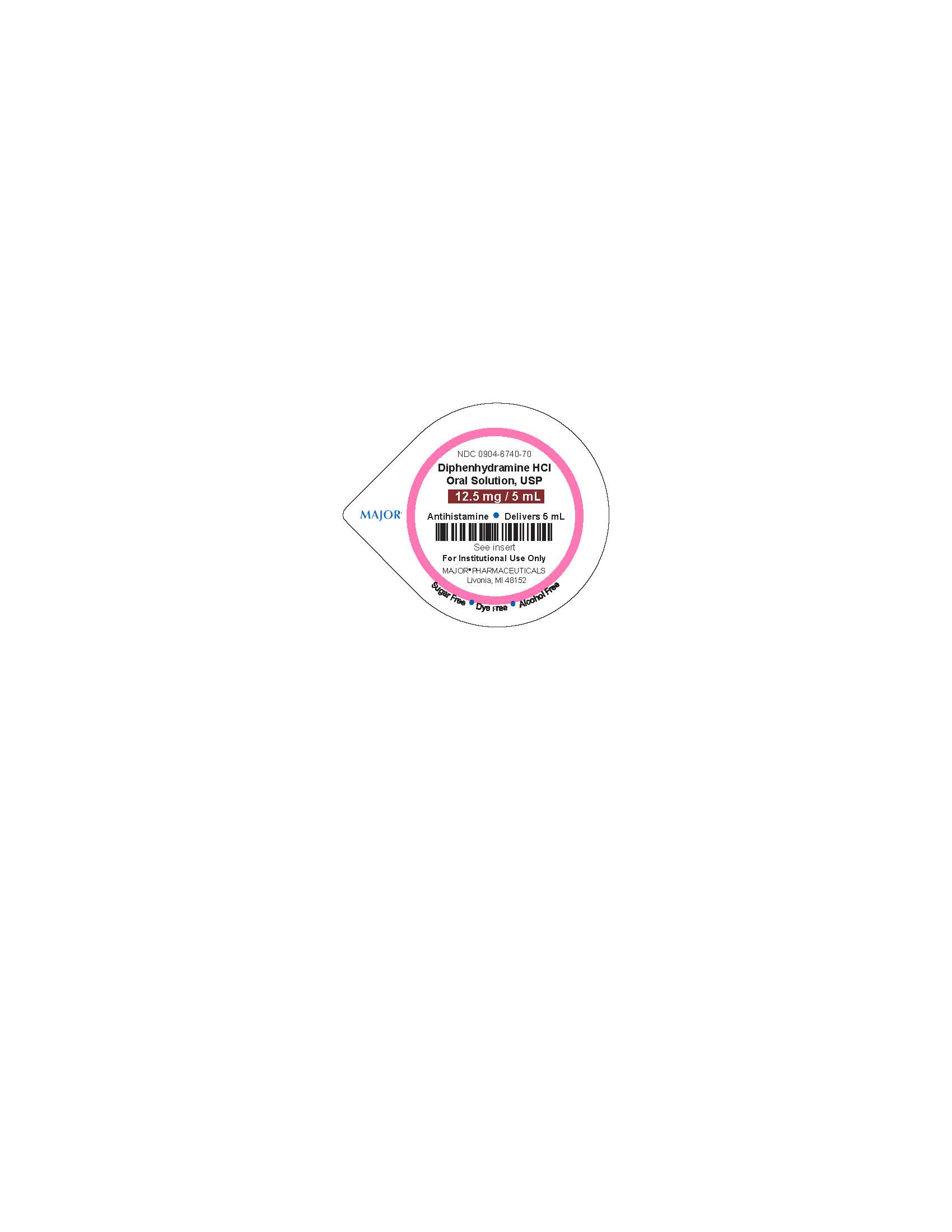 NDC: 0904-6740-70
NDC: 0904-6740-70
Diphenhydramine HCl
Oral Solution, USP
12.5 mg/5 mL
Antihistamine - Delivers 5 mL
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - Institutional Use OnlyDirections
Use the following dosage guidelines when using this product
Age (yr)
Dose (mL)
adults and children 12 years and over
take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years
take 5 mL every 4 to 6 hours; not more than 30 mL in 24 hours
children under 6 years
ask a doctor

Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - For Institutional Use OnlyWarnings
Do not use
in neonates or premature infants
if pregnant or breast-feeding
if hypersensitive to diphenhydramine HCl and other similar antihistamines
with any other product containing diphenhydramine, even one used on skin
to make a child sleepy
___________________________________________________________________
Ask a doctor before use if you have
glaucoma a breathing problem such as emphysema or chronic bronchitis
a sodium restricted diet trouble urinating due to an enlarged prostate gland
___________________________________________________________________
Ask a doctor or pharmacist before use if
taking tranquilizers or sedatives
___________________________________________________________________
When using this product
marked drowsiness may occur avoid alcoholic drinks
alcohol, sedatives, and tranquilizers may increase drowsiness
be careful when driving a motor vehicle or operating machinery
excitability may occur, especially in children
___________________________________________________________________
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - for Institutional Use OnlyInactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - For Institutional Use OnlyUses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose sneezing itchy, watery eyes itchy throat
Diphenydramine HCl 12.5 mg/ 5 mL
Major Pharmaceutical - For Institutional Use OnlyKeep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Diphenhydramine HCl 12.5 mg/ 5 mL
Major Pharmaceuticals - For Institutional Use OnlyActive ingredient (in each 5 mL cup) Purpose Diphenhydramine HCl USP 12.5 mg..………………………………………Antihistamine
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - For Institutional Use OnlyOther information
- each 5 mL contains: sodium 15 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Diphenhydramine HCl 12.5 mg/ 5 mL
Major Pharmaceuticals - IFU - For Institutional use Only
Product Insert
Diphenhydramine HCl Oral Solution, USP
NDC: 0904-6740-70
10 x 5 mL Unit Dose Cups
Active ingredient (in each 5 mL cup) Purpose Diphenhydramine HCl USP 12.5 mg..………………………………………Antihistamine
Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat
Warnings
Do not use
- in neonates or premature infants
- if pregnant or breast-feeding
- if hypersensitive to diphenhydramine HCl and other similar antihistamines
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- a sodium restricted diet
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if
- taking tranquilizers or sedatives
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Other information
- each 5 mL contains: sodium 15 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Inactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Directions
Use the following dosage guidelines when using this product
Age (yr) Dose (mL)
adults and children 12 years and over take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years take 5 mL every 4 to 6 hours; not more than 30 mL in 24 hours
children under 6 years ask a doctor
Questions or comments?
Call 1-800-616-2471
Re-order No. 700900
MAJOR® PHARMACEUTICALS
17177 N Laurel Park Dr., Suite 233
Livonia, MI 48152
-
Diphenhydramine HCl 25 mg / 10 mL Cups

 NDC: 0904-6741-72
NDC: 0904-6741-72
Diphenhydramine HCl
Oral Solution, USP
25 mg/10 mL
Antihistamine - Delivers 10 mL
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - for Instutional use OnlyDirections
Use the following dosage guidelines when using this product
Age (yr) Dose (mL)
adults and children 12 years and over take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years ask a doctor
Diphenhydramine HCl 10 mg/ 10 mL
Major Pharmaceuticals - For Institutional Use OnlyWarnings
Do not use
in neonates or premature infants
if pregnant or breast-feeding
if hypersensitive to diphenhydramine HCl and other similar antihistamines
with any other product containing diphenhydramine, even one used on skin
to make a child sleepy
___________________________________________________________________
Ask a doctor before use if you have
glaucoma a breathing problem such as emphysema or chronic bronchitis
a sodium restricted diet trouble urinating due to an enlarged prostate gland
___________________________________________________________________
Ask a doctor or pharmacist before use if
taking tranquilizers or sedatives
___________________________________________________________________
When using this product
marked drowsiness may occur avoid alcoholic drinks
alcohol, sedatives, and tranquilizers may increase drowsiness
be careful when driving a motor vehicle or operating machinery
excitability may occur, especially in children
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - For Institutional Use OnlyInactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Diphenhydramine HCl 10 mg / 10 mL
Major Pharmaceuticals - For Institutional Use OnlyUses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose sneezing itchy, watery eyes itchy throat
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - For Institutional use OnlyKeep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - For Institutional Use OnlyActive ingredient (in each 10 mL cup) Purpose Diphenhydramine HCl USP 25 mg..………………………………………Antihistamine
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - For Institutional Use Onlyeach 10 mL contains: sodium 30 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - IFU - For Institutional Use Only roduct Insert
roduct Insert
Diphenhydramine HCl Oral Solution, USP
NDC: 0904-6741-72
10 x 10 mL Unit Dose Cups
Active ingredient (in each 10 mL cup) Purpose Diphenhydramine HCl USP 25 mg..………………………………………Antihistamine
Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat
Warnings
Do not use
- in neonates or premature infants
- if pregnant or breast-feeding
- if hypersensitive to diphenhydramine HCl and other similar antihistamines
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- a sodium restricted diet
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if
- taking tranquilizers or sedatives
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
Use the following dosage guidelines when using this product
Age (yr) Dose (mL)
adults and children 12 years and over take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years ask a doctor
Other information
- each 10 mL contains: sodium 30 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Inactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Questions or comments?
Call 1-800-616-2471
Re-order
No. 700901
MAJOR® PHARMACEUTICALS
17177 N Laurel Park Dr., Suite 233
Livonia, MI 48152
-
Acetaminophen 325 mg / 10.15 mL
Major Pharmaceuticals

 NDC: 0904-6739-71
NDC: 0904-6739-71
Acetaminophen
Oral Solution, USP
325 mg / 10.15 mL
Delivers 10.15 mL
See Insert
For Institutional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 48152
Sugar Free - Dye Free - Alcohol Free
Acetaminophen 325 mg / 10.15 mL
Major PharmaceuticalsDirections
Do not use more than directed Shake well before use
Age (yr)
Dose (mL)
adults
take 20.3 mL (650 mg) every 4 to 6 hours
not to exceed 6 doses in a 24-hour period
do not use more than 10 days unless directed by a doctor
under 18 years of age
ask a doctor
Acetaminophen 325 mg / 5 mL
Major PharmaceuticalsDo not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any other inactive ingredients in this product
____________________________________________________________________________
Ask a doctor before use if the user
- has liver disease - is pregnant or breast-feeding
____________________________________________________________________________
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
____________________________________________________________________________
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days - new symptoms occur
- fever gets worse or lasts more than 3 days - redness or swelling is present
These could be signs of a serious condition
____________________________________________________________________________
Acetaminophen 325 mg / 10.15 mL
Major PharmaceuticalsInactive ingredients cherry flavor, citric acid, glycerin, methylcellulose, microcrystalline cellulose, propyl paraben, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xantham gum
Acetaminophen 325 mg / 10.15 mL
Major PharmaceuticalsActive ingredient (in each 10.15 mL cup) Purpose Acetaminophen USP 325 mg…………………………..………………..Pain reliever / fever reducer
Acetaminophen 325 mg / 10.15 mL
Major PharmaceuticalsUses temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- muscular aches
- backache
- premenstrual and menstrual cramps
- the common cold
- headache
- toothache
- temporarily reduces fever
Acetaminophen 325 mg / 10.15 mL
Major PharmaceuticalsKeep out of reach of children.
Overdose warning: taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
Acetaminophen 650 mg / 20.3 mL
Major Pharmaceuticals

 NDC: 0904-6820-76
NDC: 0904-6820-76
Acetaminophen
Oral Solution, USP
650 mg / 20.3 mL
Delivers 20.3 mL
See Insert
For Institutional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 48152
Sugar Free - Dye Free - Alcohol Free
Acetaminophen 650 mg / 20.3 mL
Major PharmaceuticalsDirections
Do not use more than directed Shake well before use
Age (yr)
Dose (mL)
adults
take 20.3 mL (650 mg) every 4 to 6 hours
not to exceed 6 doses in a 24-hour period
do not use more than 10 days unless directed by a doctor
under 18 years of age
ask a doctor
Acetaminophen 650 mg / 20.3 mL
Major PharmaceuticalsWarnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- adults take more than 6 doses in 24 hours which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
- If a skin reaction occurs, stop use and seek medical help right away
____________________________________________________________________________
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any other inactive ingredients in this product
____________________________________________________________________________
Ask a doctor before use if the user
- has liver disease
- is pregnant or breast-feeding ____________________________________________________________________________
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
____________________________________________________________________________
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- new symptoms occur
- fever gets worse or lasts more than 3 days
- redness or swelling is present
These could be signs of a serious condition
Acetaminophen 650 mg / 20.3 mL
Major PharmaceuticalsInactive ingredients cherry flavor, citric acid, glycerin, methylcellulose, microcrystalline cellulose, propyl paraben, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xantham gum
Acetaminophen 650 mg / 20.3 mL
Major PharmaceuticalsUses temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- muscular aches
- backache
- premenstrual and menstrual cramps
- the common cold
- headache
- toothache
- temporarily reduces fever
Acetaminophen 650 mg / 20.3 mL
Major PharmaceuticalsKeep out of reach of children.
Overdose warning: taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
Milk of Magnesia Concentrated 10 mL
Major Pharmaceutical OTC Monograph
NDC: 0904-6840-72
Milk of Magnesia Concentrate
2400 mg/10 mL
Magnesium Hydroxide 2400 mg.
Saline Laxative
Shake Well
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free

Milk of Magnesia Concentrated 2400 mg/ 10 mL
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
- dose may be taken once a day preferably at bedtime, in divided doses, or as directed by a doctor
- drink a full glass (8 oz) of liquid with each dose
Age (yr)
Dose (mL)
adults and children 12 years and over
10 mL, not more than 20 mL in 24 hours
children under 12 years
ask a doctor
Milk of Magnesia Concentrated 2400 mg / 10 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Milk of Magnesia concentrated 2400 mg / 10 mL
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
- stomach pain, nausea, or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
___________________________________________________________________
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
___________________________________________________________________
Stop use and ask a doctor if
- you have rectal bleeding or no bowel movements after using this product. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
___________________________________________________________________
If pregnant or breast-feeding, ask a health professional before use.
Milk of Magnesia concentrated 2400 mg / 10 mL
Inactive ingredients citric acid, glycerin, microcrystalline cellulose, methyl cellulose, purified water, saccharin sodium, sodium citrate, spearmint oil, xantham gum
Milk of Magnesia Concentrated 2400 mg / 10 mL
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in ½ to 6 hours
Milk of Magnesia Concentrated 2400 mL / 10 mL
Active ingredient (in each 10 mL cup) Magnesium hydroxide USP 2400 mg
Milk of Magnesia Concentrated 2400 mg / 10 mL
Other information
- each 10 mL contains: calcium 40 mg, sodium 35 mg, and magnesium 1000 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
-
Milk of Magnesia 2400 mg/30 mL
Major Pharmaceutical OTC Monograph
 NDC: 0904-6846-73
NDC: 0904-6846-73
Milk of Magnesia USP
2400 mg/30 mL
Magnesium Hydroxide 2400 mg.
Saline Laxative
Shake Well
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
Milk of Magnesia 2400 mg/30 mL
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
- dose may be taken once a day preferably at bedtime, or as directed by a doctor
- drink a full glass (8 oz) of liquid with each dose
Age (yr)
Dose (mL)
adults and children 12 years and over
30 mL, not more than 60 mL in 24 hrs.
children under 12 years
ask a doctor
Milk of Magnesia 2400 mg/ 30 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Milk of Magnesia 2400 mg/ 30 mL
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
- stomach pain, nausea, or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
___________________________________________________________________
Stop use and ask a doctor if
- you have rectal bleeding or no bowel movements after using this product. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
___________________________________________________________________
If pregnant or breast-feeding, ask a health professional before use.
Milk of Magnesia 2400 mg / 30 mL
Inactive ingredients citric acid, glycerin, microcrystalline cellulose, methyl cellulose, purified water, saccharin sodium, sodium citrate, spearmint oil, xanthan gum
Milk of Magnesia 2400 mg / 30 mL
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in ½ to 6 hours
Milk of Magnesia 2400 mg / 30 mL
Active ingredient (in each 30 mL cup)
Magnesium hydroxide USP 2400 mg
Milk of Magnesia 2400 mg / 30 mL
Other information
- each 30 mL contains: calcium 40 mg, sodium 100 mg, and magnesium 1000 mg
- store at 20-25°C (68-77°F) -
- protect from excessive moisture
- do not use if lid seal is open or damaged -
sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN ORAL SOLUTION
acetaminophen oral solution solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6739 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 10.15 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SORBITOL (UNII: 506T60A25R) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) METHYLCELLULOSE (15 CPS) (UNII: NPU9M2E6L8) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) Product Characteristics Color white (white to light pink) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6739-71 10 in 1 CASE 04/08/2019 1 10 in 1 TRAY 1 10.15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/08/2019 DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6740 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) POLOXAMER 407 (UNII: TUF2IVW3M2) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6740-70 10 in 1 CASE 12/04/2018 1 10 in 1 TRAY 1 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/04/2018 DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6741 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) POLOXAMER 407 (UNII: TUF2IVW3M2) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6741-72 10 in 1 CASE 12/04/2018 1 10 in 1 TRAY 1 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/04/2018 ACETAMINOPHEN ORAL SOLUTION
acetaminophen oral solution solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6738 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SORBITOL (UNII: 506T60A25R) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) METHYLCELLULOSE (15 CPS) (UNII: NPU9M2E6L8) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) Product Characteristics Color white (White to light pink) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6738-70 10 in 1 CASE 04/08/2019 1 10 in 1 TRAY 1 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/08/2019 ACETAMINOPHEN ORAL SOLUTION
acetaminophen oral solution solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6820 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20.3 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SORBITOL (UNII: 506T60A25R) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) METHYLCELLULOSE (15 CPS) (UNII: NPU9M2E6L8) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) Product Characteristics Color white (white to light pink) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6820-76 10 in 1 CASE 04/08/2019 1 10 in 1 TRAY 1 20.3 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/08/2019 MILK OF MAGNESIA
magnesium hydroxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6840 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (HYDROXIDE ION - UNII:9159UV381P, MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 2400 mg in 10 mL Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) METHYLCELLULOSE (15 CPS) (UNII: NPU9M2E6L8) SUCRALOSE (UNII: 96K6UQ3ZD4) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (Suspension) Score Shape Size Flavor SPEARMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6840-72 10 in 1 CASE 06/10/2019 1 10 in 1 TRAY 1 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 06/10/2019 MILK OF MAGNESIA
magnesium hydroxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6846 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (HYDROXIDE ION - UNII:9159UV381P, MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 2400 mg in 30 mL Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) METHYLCELLULOSE (15 CPS) (UNII: NPU9M2E6L8) SUCRALOSE (UNII: 96K6UQ3ZD4) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (Suspension) Score Shape Size Flavor SPEARMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6846-73 10 in 1 CASE 06/10/2019 1 10 in 1 TRAY 1 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 06/10/2019 Labeler - Major Pharmaceuticals (191427277) Registrant - Plastikon Healthcare, LLC (041717941) Establishment Name Address ID/FEI Business Operations Plastikon Healthcare, LLC 041717941 manufacture(0904-6740, 0904-6741, 0904-6738, 0904-6739, 0904-6820, 0904-6840, 0904-6846)
Trademark Results [Milk of Magnesia]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MILK OF MAGNESIA 70024049 0024049 Dead/Cancelled |
Charles H. Phillips Chemical Company ThE 1893-12-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.




