AKEEGA- niraparib tosylate monohydrate and abiraterone acetate tablet, film coated
AKEEGA by
Drug Labeling and Warnings
AKEEGA by is a Prescription medication manufactured, distributed, or labeled by Janssen Biotech, Inc., Shanghai SynTheAll Pharmaceutical Co., Ltd., Changzhou SynTheAll Pharmaceutical Co., Ltd., Jiangsu Jiaerke Pharmaceuticals Group Corp., Ltd., Zhejiang Xianju Junye Pharmaceutical Co., Ltd., Pharmacia & Upjohn Company LLC, Janssen Pharmaceutica NV, Patheon France S.A.S, Janssen Cilag SpA, Johnson & Johnson Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AKEEGA safely and effectively. See full prescribing information for AKEEGA.
AKEEGA ® (niraparib and abiraterone acetate) tablets, for oral use
Initial U.S. Approval: 2023RECENT MAJOR CHANGES
INDICATIONS AND USAGE
AKEEGA is a combination of niraparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, and abiraterone acetate, a CYP17 inhibitor indicated with prednisone for the treatment of adult patients with:
- deleterious or suspected deleterious BRCA2-mutated ( BRCA2m) metastatic castration-sensitive prostate cancer (mCSPC).
- deleterious or suspected deleterious BRCA-mutated ( BRCAm) metastatic castration-resistant prostate cancer (mCRPC).
- Select patients for therapy based on an FDA-approved test for AKEEGA. ( 1, 2.1)
DOSAGE AND ADMINISTRATION
BRCA2m mCSPC:
- The recommended dosage of AKEEGA is 200 mg niraparib/1,000 mg abiraterone acetate orally once daily in combination with 5 mg prednisone daily until disease progression or unacceptable toxicity. ( 2.2)
BRCAm mCRPC :
- The recommended dosage of AKEEGA is 200 mg niraparib/1,000 mg abiraterone acetate orally once daily in combination with 10 mg prednisone daily until disease progression or unacceptable toxicity. ( 2.2)
- Patients receiving AKEEGA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy. ( 2.2)
- Take AKEEGA on an empty stomach at least one hour before or two hours after food. ( 2.2)
- For adverse reactions, consider interruption of treatment, dose reduction, or dose discontinuation. ( 2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML): MDS/AML, including a case with fatal outcome, has been observed in patients treated with AKEEGA. Monitor patients for hematological toxicity and discontinue if MDS/AML is confirmed. ( 5.1)
- Myelosuppression:Test complete blood counts weekly for the first month, every two weeks for the next two months, monthly for the remainder of the first year, then every other month, and as clinically indicated. ( 2.3, 5.2)
- Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions:Monitor patients for hypertension, hypokalemia, and fluid retention at least weekly for the first two months, then once a month. Closely monitor patients whose underlying medical conditions might be compromised by increases in blood pressure, hypokalemia, or fluid retention. Control hypertension and correct hypokalemia before and during treatment with AKEEGA. ( 5.3)
- Hepatotoxicity:Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue treatment as recommended. ( 2.3, 5.4)
- Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during and after stressful situations. ( 5.5)
- Hypoglycemia:Severe hypoglycemia has been reported when abiraterone acetate, a component of AKEEGA, was administered to patients receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide. Monitor blood glucose in patients with diabetes during and assess if antidiabetic agent dose modifications are required. ( 5.6)
- Increased fractures and mortality in combination with radium Ra 223 dichloride: Use of AKEEGA plus prednisone in combination with radium Ra 223 dichloride is not recommended. ( 5.7)
- Posterior Reversible Encephalopathy Syndrome (PRES):PRES has been observed in patients treated with niraparib, a component of AKEEGA. Discontinue AKEEGA if PRES is confirmed. ( 5.8)
- Embryo-Fetal Toxicity:AKEEGA can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception. ( 5.9, 8.1, 8.3)
ADVERSE REACTIONS
- The most common adverse reactions (≥20%), including laboratory abnormalities, are decreased hemoglobin, decreased lymphocytes, musculoskeletal pain, fatigue, decreased platelets, increased alkaline phosphatase, constipation, hypertension, nausea, decreased neutrophils, increased creatinine, increased potassium, decreased potassium, increased AST, fluid retention/edema, increased bilirubin, respiratory tract infection and arrhythmia. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Strong CYP3A4 Inducers: Avoid coadministration. ( 7.1)
- CYP2D6 Substrates: Avoid coadministration of AKEEGA with CYP2D6 substrates for which minimal changes in concentration may lead to serious toxicities. If alternative treatments cannot be used, consider a dose reduction of the concomitant CYP2D6 substrate. ( 7.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage
2.3 Dosage Modification for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
5.2 Myelosuppression
5.3 Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions
5.4 Hepatotoxicity
5.5 Adrenocortical Insufficiency
5.6 Hypoglycemia
5.7 Increased Fractures and Mortality in Combination with Radium 223 Dichloride
5.8 Posterior Reversible Encephalopathy Syndrome
5.9 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on AKEEGA
7.2 Effects of AKEEGA on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 BRCA2-mutated Metastatic Castration-Sensitive Prostate Cancer (mCSPC)
14.2 BRCA-mutated Metastatic Castration-Resistant Prostate Cancer (mCRPC)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
AKEEGA with prednisone is indicated for the treatment of adult patients with deleterious or suspected deleterious BRCA2-mutated ( BRCA2m) metastatic castration-sensitive prostate cancer (mCSPC).
AKEEGA with prednisone is indicated for the treatment of adult patients with deleterious or suspected deleterious BRCA-mutated ( BRCAm) metastatic castration-resistant prostate cancer (mCRPC).
Select patients for therapy based on an FDA-approved test for AKEEGA [see Dosage and Administration (2.1)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of mCSPC with AKEEGA based on the presence of a BRCA2 gene alteration [see Clinical Studies (14.1)] .
Select patients for the treatment of mCRPC with AKEEGA based on the presence of a BRCA gene alteration [see Clinical Studies (14.2)] .
Information on FDA-approved tests is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
BRCA2-mutated ( BRCA2m) Metastatic Castration-Sensitive Prostate Cancer (mCSPC)
- The recommended dosage of AKEEGA is 200 mg niraparib/1,000 mg abiraterone acetate orally once daily in combination with 5 mg prednisone once daily until disease progression or unacceptable toxicity.
BRCA-mutated ( BRCAm) Metastatic Castration-Resistant Prostate Cancer (mCRPC)
- The recommended dosage of AKEEGA is 200 mg niraparib/1,000 mg abiraterone acetate orally once daily in combination with 10 mg prednisone once daily until disease progression or unacceptable toxicity.
Patients receiving AKEEGA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy.
Take AKEEGA on an empty stomach at least one hour before or two hours after food. Swallow tablets whole with water. Do not break, crush, or chew tablets.
If a patient misses a dose, instruct patients to take the dose as soon as possible on the same day and resume their next dose at the normal schedule the following day.
2.3 Dosage Modification for Adverse Reactions
The recommended dosage modifications for AKEEGA are provided in Table 1.
Treatment with AKEEGA should not be reinitiated until the toxicity has resolved to Grade 1 or baseline. If the toxicity is attributed to one component of AKEEGA, the other component of AKEEGA may be continued as a single agent at the current dose until the adverse reaction resolves and AKEEGA can be resumed (see Table 1).
Table 1: Dosage Modifications for Adverse Reactions Adverse Reaction Severity Dosage Modification - * If myelodysplastic syndrome or acute myeloid leukemia (MDS/AML) is confirmed, discontinue AKEEGA [see Warnings and Precautions (5.1)].
- † Discontinue AKEEGA in patients who develop hypertensive crisis or other severe cardiovascular adverse reactions [see Warnings and Precautions (5.3)].
Myelosuppression
[see Warnings and Precautions (5.2)]Hemoglobin <8 g/dL - Withhold AKEEGA and monitor blood counts weekly.
- When hemoglobin returns to ≥9 g/dL, resume at the reduced dose of AKEEGA 100 mg/1,000 mg once daily and monitor blood counts weekly for 28 days and as clinically indicated.
- Permanently discontinue AKEEGA if hemoglobin has not returned to acceptable levels within 28 days of the dose interruption period or if the patient has already undergone dose reduction to 100 mg/1,000 mg once daily. *
Platelet count <100,000/mcL First occurrence: - Withhold AKEEGA for a maximum of 28 days and monitor blood counts weekly until platelet counts return to ≥100,000/mcL.
- Resume AKEEGA at same or the reduced dose of 100 mg/1,000 mg once daily.
- If platelet count is <75,000/mcL, resume at the reduced dose of AKEEGA 100 mg/1,000 mg once daily.
- Withhold AKEEGA for a maximum of 28 days and monitor blood counts weekly until platelet counts return to ≥100,000/mcL.
- Resume at the reduced dose of AKEEGA 100 mg/1,000 mg once daily.
- Permanently discontinue AKEEGA if the platelet count has not returned to acceptable levels within 28 days of the dose interruption period or if the patient has already undergone dose reduction to 100 mg/1,000 mg once daily. *
Neutrophil <1,000/mcL - Withhold AKEEGA and monitor blood counts weekly.
- When neutrophil counts return to ≥1,500/mcL, resume at the reduced dose of AKEEGA 100 mg/1,000 mg once daily and monitor blood counts weekly for 28 days and as clinically indicated.
- Permanently discontinue AKEEGA if neutrophils have not returned to acceptable levels within 28 days of the dose interruption period or if the patient has already undergone dose reduction to 100 mg/1,000 mg once daily. *
Hematologic adverse reaction requiring transfusion - Consider platelet transfusion for patients with platelet count ≤10,000/mcL. If there are other risk factors such as coadministration of anticoagulation or antiplatelet drugs, consider interrupting these drugs and/or transfusion at a higher platelet count.
- Resume at the reduced dose of AKEEGA 100 mg/1,000 mg once daily.
Hepatotoxicity
[see Warnings and Precautions (5.4)]ALT and/or AST greater than 5 × ULN or total bilirubin greater than 3 × ULN - Withhold AKEEGA and closely monitor liver function.
- Permanently discontinue AKEEGA if:
ALT or AST ≥ 20 times the ULN
– OR–
ALT > 3 × ULN and total bilirubin > 2 × ULN in the absence of biliary obstruction or other causes responsible for the concurrent elevation
-OR-
Hepatotoxicity recurs at the reduced dose 100 mg/500 mg. - When AST and ALT resolves to less ≤ 2.5 × ULN and total bilirubin ≤ 1.5 × ULN, AKEEGA may be resumed at the reduced dose of 100 mg/500 mg once daily. When resumed, monitor serum transaminases every two weeks for three months, monthly thereafter, and as clinically indicated.
Other non-hematological adverse reactions that persist despite medical management [see Warnings and Precautions (5)and Adverse Reactions (6.1)] Grade 3 or 4 † - Withhold AKEEGA until resolution of adverse reaction or for a maximum of 28 days.
- If resolves in 28 days or less, AKEEGA may be resumed at the reduced dose.
- Permanently discontinue AKEEGA if adverse reaction(s) has not resolved after 28 days or Grade 3 or 4 adverse reaction reoccurs after dose reduction.
-
3 DOSAGE FORMS AND STRENGTHS
Tablets
- 50 mg niraparib/500 mg abiraterone acetate: yellowish orange to yellowish brown, oval, film-coated tablets debossed with "N 50 A" on one side and plain on the other side.
- 100 mg niraparib/500 mg abiraterone acetate: orange, oval, film-coated tablets debossed with "N 100 A" on one side and plain on the other side.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
AKEEGA may cause myelodysplastic syndrome/acute myeloid leukemia (MDS/AML).
In the individual AMPLITUDE and MAGNITUDE studies, MDS or AML, including cases with fatal outcomes, were reported in 0.6% (2/347) and 0.5% (1/212) of patients treated with AKEEGA plus prednisone, respectively.
All patients in other tumor types treated with niraparib, a component of AKEEGA, who developed secondary MDS/cancer-therapy-related AML had received previous chemotherapy with platinum agents and/or other DNA-damaging agents, including radiotherapy.
For suspected MDS/AML or prolonged hematological toxicities, refer the patient to a hematologist for further evaluation. Discontinue AKEEGA if MDS/AML is confirmed.
5.2 Myelosuppression
AKEEGA may cause myelosuppression (anemia, thrombocytopenia, or neutropenia).
In AMPLITUDE, Grade 3–4 anemia, neutropenia, and thrombocytopenia were reported, respectively in 29%, 10%, and 4.9% of patients receiving AKEEGA. Overall, 25% of patients with anemia required a red blood cell transfusion, including 15% who required more than one transfusion. Discontinuation due to anemia occurred in 1.2% of patients.
In MAGNITUDE Cohort 1, Grade 3–4 anemia, thrombocytopenia, and neutropenia were reported, respectively in 28%, 8%, and 7% of patients receiving AKEEGA. Overall, 27% of patients with anemia required a red blood cell transfusion, including 19.5% who required more than one transfusion. Discontinuation due to anemia occurred in 3% of patients.
Monitor complete blood counts weekly during the first month of AKEEGA treatment, every two weeks for the next two months, monthly for the remainder of the first year and then every other month, and as clinically indicated. Do not start AKEEGA until patients have adequately recovered from hematologic toxicity caused by previous therapy. If hematologic toxicities do not resolve within 28 days following interruption, discontinue AKEEGA and refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics [see Dosage and Administration (2.3)] .
5.3 Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions
AKEEGA may cause hypokalemia and fluid retention as a consequence of increased mineralocorticoid levels resulting from CYP17 inhibition [see Clinical Pharmacology (12.1)] . In post-marketing experience, QT prolongation and Torsades de Pointes have been observed in patients who develop hypokalemia while taking abiraterone acetate, a component of AKEEGA. Hypertension and hypertensive crisis have also been reported in patients treated with niraparib, a component of AKEEGA.
In AMPLITUDE, which used prednisone 5 mg daily in combination with AKEEGA, Grades 3–4 hypokalemia was detected in 9% of patients on the AKEEGA arm, and Grades 3–4 hypertension was observed in 30% of patients on the AKEEGA arm.
In MAGNITUDE Cohort 1, which used prednisone 10 mg daily in combination with AKEEGA, Grade 3–4 hypokalemia was detected in 2.7% of patients on the AKEEGA arm and Grade 3–4 hypertension was observed in 14% of patients on the AKEEGA arm.
Monitor patients for hypertension, hypokalemia, and fluid retention at least weekly for the first two months, then once a month. Closely monitor patients whose underlying medical conditions might be compromised by increases in blood pressure, hypokalemia, or fluid retention, such as those with heart failure, recent myocardial infarction, cardiovascular disease, or ventricular arrhythmia. Control hypertension and correct hypokalemia before and during treatment with AKEEGA. Discontinue AKEEGA in patients who develop hypertensive crisis or other severe cardiovascular adverse reactions.
The safety of AKEEGA in patients with New York Heart Association (NYHA) Class II to IV heart failure has not been established because these patients were excluded from AMPLITUDE and MAGNITUDE.
5.4 Hepatotoxicity
AKEEGA may cause hepatotoxicity.
Hepatotoxicity in patients receiving abiraterone acetate, a component of AKEEGA, has been reported in clinical trials. In post-marketing experience, there have been abiraterone acetate-associated severe hepatic toxicity, including fulminant hepatitis, acute liver failure, and deaths.
In AMPLITUDE, Grade 3–4 ALT or AST increases (at least 5 × ULN) were reported in 1.9% and 1.3% of patients, respectively.
In MAGNITUDE Cohort 1, Grade 3–4 ALT or AST increases (at least 5 × ULN) were reported in 1.8% and 0.9% of patients, respectively.
The safety of AKEEGA in patients with moderate or severe hepatic impairment has not been established as these patients were excluded from AMPLITUDE and MAGNITUDE.
Measure serum transaminases (ALT and AST) and bilirubin levels prior to starting treatment with AKEEGA, every two weeks for the first three months of treatment and monthly thereafter. Promptly measure serum total bilirubin, AST, and ALT if clinical symptoms or signs suggestive of hepatotoxicity develop. Elevations of AST, ALT, or bilirubin from the patient's baseline should prompt more frequent monitoring and may require dosage modifications [see Dosage and Administration (2.3)].
Permanently discontinue AKEEGA for patients who develop a concurrent elevation of ALT greater than 3 × ULN and total bilirubin greater than 2 × ULN in the absence of biliary obstruction or other causes responsible for the concurrent elevation, or in patients who develop ALT or AST ≥20 × ULN at any time after receiving AKEEGA.
5.5 Adrenocortical Insufficiency
AKEEGA may cause adrenal insufficiency.
Adrenocortical insufficiency has been reported in clinical trials in patients receiving abiraterone acetate, a component of AKEEGA, in combination with prednisone, following interruption of daily steroids and/or with concurrent infection or stress. Monitor patients for symptoms and signs of adrenocortical insufficiency, particularly if patients are withdrawn from prednisone, have prednisone dose reductions, or experience unusual stress. Symptoms and signs of adrenocortical insufficiency may be masked by adverse reactions associated with mineralocorticoid excess seen in patients treated with abiraterone acetate. If clinically indicated, perform appropriate tests to confirm the diagnosis of adrenocortical insufficiency. Increased doses of corticosteroids may be indicated before, during, and after stressful situations.
5.6 Hypoglycemia
AKEEGA may cause hypoglycemia in patients being treated with other medications for diabetes.
Severe hypoglycemia has been reported when abiraterone acetate, a component of AKEEGA, was administered to patients receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide [see Drug Interactions (7.2)] .
Monitor blood glucose in patients with diabetes during and after discontinuation of treatment with AKEEGA. Assess if antidiabetic drug dosage needs to be adjusted to minimize the risk of hypoglycemia.
5.7 Increased Fractures and Mortality in Combination with Radium 223 Dichloride
AKEEGA with prednisone is not recommended for use in combination with Ra-223 dichloride outside of clinical trials.
The clinical efficacy and safety of concurrent initiation of abiraterone acetate plus prednisone/prednisolone and radium Ra 223 dichloride was assessed in a randomized, placebo-controlled multicenter study (ERA-223 trial) in 806 patients with asymptomatic or mildly symptomatic castration-resistant prostate cancer with bone metastases. The study was unblinded early based on an Independent Data Monitoring Committee recommendation.
At the primary analysis, increased incidences of fractures (29% vs 11%) and deaths (39% vs 36%) have been observed in patients who received abiraterone acetate plus prednisone/prednisolone in combination with radium Ra 223 dichloride compared to patients who received placebo in combination with abiraterone acetate plus prednisone.
It is recommended that subsequent treatment with Ra-223 not be initiated for at least five days after the last administration of AKEEGA, in combination with prednisone.
5.8 Posterior Reversible Encephalopathy Syndrome
AKEEGA may cause Posterior Reversible Encephalopathy Syndrome (PRES).
PRES has been observed in patients treated with niraparib as a single agent at higher than the recommended dose of niraparib included in AKEEGA.
Monitor all patients treated with AKEEGA for signs and symptoms of PRES. If PRES is suspected, promptly discontinue AKEEGA and administer appropriate treatment. The safety of reinitiating AKEEGA in patients previously experiencing PRES is not known.
5.9 Embryo-Fetal Toxicity
The safety and efficacy of AKEEGA have not been established in females. Based on animal reproductive studies and mechanism of action, AKEEGA can cause fetal harm and loss of pregnancy when administered to a pregnant female [see Clinical Pharmacology (12.1)] .
Niraparib has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow) [see Warnings and Precautions (5.2)and Nonclinical Toxicology (13.1)] .
In animal reproduction studies, oral administration of abiraterone acetate to pregnant rats during organogenesis caused adverse developmental effects at maternal exposures approximately ≥ 0.03 times the human exposure (AUC) at the recommended dose.
Advise males with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of AKEEGA [see Use in Specific Populations (8.1, 8.3)] . Females who are or may become pregnant should handle AKEEGA with protection, e.g., gloves [see How Supplied/Storage and Handling (16)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling:
- Myelodysplastic syndrome/acute myeloid leukemia [see Warnings and Precautions (5.1)]
- Myelosuppression [see Warnings and Precautions (5.2)]
- Hypokalemia, fluid retention, and cardiovascular adverse reactions [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Adrenocortical insufficiency [see Warnings and Precautions (5.5)]
- Hypoglycemia [see Warnings and Precautions (5.6)]
- Increased fractures and mortality in combination with Radium 223 Dichloride [see Warnings and Precautions (5.7)]
- Posterior reversible encephalopathy syndrome [see Warnings and Precautions (5.8)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety population described in the WARNINGS and PRECAUTIONS reflect exposure to AKEEGA (niraparib 200 mg and abiraterone acetate 1,000 mg) in BRCA2m patients (N=162) in the AMPLITUDE study and in BRCAm patients in Cohort 1 (N=113) in the MAGNITUDE study unless otherwise specified.
BRCA2-mutated Metastatic Castration-Sensitive Prostate Cancer (mCSPC)
The safety of AKEEGA in patients with BRCA2m mCSPC was evaluated in AMPLITUDE [see Clinical Studies (14.1)]. Patients were randomized to receive either AKEEGA (niraparib 200 mg and abiraterone acetate 1,000 mg once daily) (n=162), or placebo and abiraterone acetate (n=161) until unacceptable toxicity or progression. Patients in both arms also received prednisone 5 mg daily. The median duration of exposure for AKEEGA was 26 months (range: 0 to 48 months).
Serious adverse reactions occurred in 36% of patients who received AKEEGA. Serious adverse reactions reported in >2% of patients included anemia (4.9%), and pneumonia (3.7%). Fatal adverse reactions occurred in 4.9% of patients who received AKEEGA, including sudden death (1.9%), COVID-19 pneumonia (1.2%), pneumocystis jirovecii pneumonia (0.6%), pneumonia (0.6%), and cardio-respiratory arrest (0.6%).
Permanent discontinuation of any component of AKEEGA due to an adverse reaction occurred in 13% of patients.
Dosage interruptions of any component of AKEEGA due to an adverse reaction occurred in 67% of patients. Adverse reactions which required dosage interruption in >2% of patients included anemia (30%), COVID-19 (10%), hypertension (9%), neutropenia (8%), thrombocytopenia (8%), hypokalemia (7%), vomiting (4.9%), fatigue (4.3%), diarrhea (2.5%), and pneumonia (2.5%).
Dose reductions of any component of AKEEGA due to an adverse reaction occurred in 25% of patients. Adverse reactions which required dose reductions in >2% of patients included anemia (17%).
The most common adverse reactions (>20%), including laboratory abnormalities, in patients who received AKEEGA were decreased hemoglobin, decreased lymphocyte count, hypertension, decreased neutrophil count, musculoskeletal pain, decreased platelet count, constipation, fatigue, decreased potassium, increase creatinine, nausea, increased alkaline phosphate, increased aspartate aminotransferase, respiratory tract infection, arrhythmia, increased blood bilirubin, and fluid retention/edema.
Table 2: Adverse Reactions (>20%) in Patients with BRCA2m mCSPC Who Received AKEEGA (with a Difference of ≥5% Compared to Placebo) in AMPLITUDE Adverse Reaction AKEEGA
(N=162)Placebo with Abiraterone Acetate
(N=161)All Grades
%Grade 3 or 4
%All Grades
%Grade 3 or 4
%- * Grouped terms including multiple similar terms
Vascular disorders Hypertension * 51 31 36 19 Musculoskeletal and connective tissue disorders Musculoskeletal pain * 45 6 58 4.3 Gastrointestinal disorders Constipation 41 0 17 0.6 Nausea 30 0 17 0 General disorders and administration Fatigue * 39 4.3 29 3.1 Respiratory, thoracic and mediastinal disorders Respiratory Tract Infection * 23 0.6 13 0.6 Cardiac disorders Arrhythmia * 23 3.7 9 2.5 Clinically relevant adverse reactions that occurred in ≤20% of patients receiving AKEEGA plus prednisone were hot flush (18%), vomiting (17%), dizziness (17%), abdominal pain (15%), weight decreased (14%), diarrhea (14%), decreased appetite (12%), headache (12%), hemorrhage (12%), dyspnea (10%), urinary tract infection (8%), pneumonia (7%), osteoporosis (4.9%), rash (3.7%), cardiac failure (3.1%), ischemic heart disease (4.9%), acute kidney injury (2.5%), pulmonary embolism (2.5%), and urosepsis (0.6%).
The most common select laboratory abnormalities (>20%) that worsened from baseline in patients who received AKEEGA are in Table 3.
Table 3: Select Laboratory Abnormalities (>20%) That Worsened from Baseline in Patients with BRCA2m mCSPC Who Received AKEEGA in AMPLITUDE Laboratory Abnormality AKEEGA *
(N=162)Placebo with Abiraterone Acetate *
(N=161)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate varied from 160 to 161 for placebo with abiraterone acetate plus prednisone and 159 to 162 for AKEEGA with prednisone based on the number of patients with a baseline value and at least one post-treatment value.
Hematology Decreased Hemoglobin 74 29 53 1.9 Decreased Lymphocyte Count 59 20 37 13 Decreased Neutrophil Count 49 10 19 3.1 Decreased Platelet Count 41 4.9 23 0.6 Chemistry Decreased Potassium 38 9 29 10 Increased Creatinine 30 1.3 16 2.5 Increased Alkaline Phosphatase 28 0.6 24 3.1 Increased Aspartate Aminotransferase 24 1.3 33 2.5 Increased Blood Bilirubin 22 0 11 0 BRCA-mutated Metastatic Castration-Resistant Prostate Cancer
The safety of AKEEGA in patients with BRCAm mCRPC was evaluated in Cohort 1 of MAGNITUDE [see Clinical Studies (14.2)]. Patients were randomized to receive either AKEEGA (niraparib 200 mg and abiraterone acetate 1,000 mg once daily) (n=113), or placebo and abiraterone acetate (n=112) until unacceptable toxicity or progression. Patients in both arms also received prednisone 10 mg daily. The median duration of exposure for AKEEGA was 18 months (range: 0 to 37 months).
Serious adverse reactions occurred in 41% of patients who received AKEEGA. Serious adverse reactions reported in >2% of patients included COVID-19 (7%), anemia (4.4%), pneumonia (3.5%), and hemorrhage (3.5%). Fatal adverse reactions occurred in 9% of patients who received AKEEGA, including COVID-19 (5%), cardiopulmonary arrest (1%), dyspnea (1%), pneumonia (1%), and septic shock (1%).
Permanent discontinuation of any component of AKEEGA due to an adverse reaction occurred in 15% of patients. Adverse reactions which resulted in permanent discontinuation of AKEEGA in > 2% of patients included COVID-19 (4.4%), anemia (2.7%), asthenia (2.7%), and vomiting (2.7%).
Dosage interruptions of any component of AKEEGA due to an adverse reaction occurred in 50% of patients. Adverse reactions which required dosage interruption in > 2% of patients included anemia (23%), thrombocytopenia (12%), neutropenia (7%), COVID-19 (6%), fatigue (3.5%), asthenia (3.5%), nausea (3.5%), pneumonia (2.7%), hematuria (2.7%), and vomiting (2.7%).
Dose reductions of any component of AKEEGA due to an adverse reaction occurred in 28% of patients. Adverse reactions which required dose reductions in > 2% of patients included anemia (12%), thrombocytopenia (4.4%), and fatigue (2.7%).
The most common adverse reactions (>20%), including laboratory abnormalities, in patients who received AKEEGA were hemoglobin decreased, lymphocyte decreased, musculoskeletal pain, fatigue, platelets decreased, constipation, alkaline phosphatase increased, hypertension, nausea, neutrophils decreased, creatinine increased, potassium increased, potassium decreased, and aspartate aminotransferase increased.
Tables 4 and 5 summarize adverse reactions and laboratory abnormalities for patients with BRCAm mCRPC in MAGNITUDE, respectively.
Table 4: Adverse Reactions (>10%) in Patients with BRCAm mCRPC Who Received AKEEGA in MAGNITUDE AKEEGA
(N=113)Placebo with Abiraterone Acetate
(N=112)Adverse Reaction All Grades
%Grade 3 or 4
%All Grades
%Grade 3 or 4
%- * Grouped terms including multiple similar terms.
Musculoskeletal and connective tissue disorders Musculoskeletal pain * 44 4 42 5 General disorders and administration site conditions Fatigue * 43 5 30 4 Edema * 17 0 9 0 Pyrexia * 10 2 6 0 Gastrointestinal disorders Constipation 34 1 20 0 Vomiting 15 0 7 1 Nausea 33 1 21 0 Abdominal pain * 12 2 12 1 Vascular disorders Hypertension * 33 14 27 17 Hemorrhage * 12 2 8 1 Respiratory, thoracic and mediastinal disorders Dyspnea * 15 1 8 3 Cough * 12 0 6 0 Metabolism and nutrition disorders Decreased appetite 15 2 8 0 Nervous system disorders Dizziness * 14 0 10 0 Headache 12 1 9 0 Infections and infestations COVID-19 * 13 7 9 4 Urinary tract infection * 12 3 9 1 Psychiatric disorders Insomnia 12 0 4 0 Investigations Weight decreased 10 1 4 1 Cardiac disorders Arrhythmia * 10 2 4 1 Injury, poisoning and procedural complications Fall 10 1 13 4 Clinically relevant adverse events that occurred in <10% of patients receiving AKEEGA plus prednisone were rash (7%), alanine aminotransferase increased (5%), aspartate aminotransferase increased (5%), cerebrovascular accident (4.4%), pulmonary embolism (2.7%), deep vein thrombosis (2.7%), and acute kidney injury (2.7%).
Table 5: Select Laboratory Abnormalities (>20%) That Worsened from Baseline in Patients with BRCAm mCRPC Who Received AKEEGA in MAGNITUDE AKEEGA *
(N=113)Placebo with Abiraterone Acetate *
(N=112)Laboratory Abnormality All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate varied from 111 to 112 for placebo with abiraterone acetate plus prednisone and 113 for AKEEGA with prednisone based on the number of patients with a baseline value and at least one post-treatment value.
Hematology Hemoglobin decreased 67 26 53 7 Lymphocyte decreased 55 22 32 13 Platelets decreased 37 8 22 1.8 Neutrophils decreased 32 7 16 2.7 Chemistry Alkaline Phosphatase increased 34 1.8 29 1.8 Creatinine increased 30 0 13 1.8 Potassium increased 25 0.9 21 3.6 Potassium decreased 20 5 20 5 Aspartate Aminotransferase increased 20 1.8 25 2.7 Other Clinical Trial Experience
The following adverse reactions have been reported with the individual components of AKEEGA but were not observed in AMPLITUDE or MAGNITUDE Cohort 1: myopathy, rhabdomyolysis, adrenal insufficiency, allergic alveolitis, febrile neutropenia, anaphylactic reaction, posterior reversible encephalopathy (PRES), and hypertensive crisis.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on AKEEGA
Effect of CYP3A4 Inducers
Avoid coadministration with strong CYP3A4 inducers [see Clinical Pharmacology (12.3)] .
Abiraterone is a substrate of CYP3A4. Strong CYP3A4 inducers may decrease abiraterone concentrations [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of abiraterone.
7.2 Effects of AKEEGA on Other Drugs
CYP2D6 Substrates
Avoid coadministration unless otherwise recommended in the Prescribing Information for CYP2D6 substrates for which minimal changes in concentration may lead to serious toxicities. If alternative treatments cannot be used, consider a dose reduction of the concomitant CYP2D6 substrate drug.
Abiraterone is a CYP2D6 moderate inhibitor. AKEEGA increases the concentration of CYP2D6 substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates.
CYP2C8 Substrates
Monitor patients for signs of toxicity related to a CYP2C8 substrate for which a minimal change in plasma concentration may lead to serious or life-threatening adverse reactions.
Abiraterone is a CYP2C8 inhibitor. AKEEGA increases the concentration of CYP2C8 substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The safety and efficacy of AKEEGA have not been established in females. Based on findings from animal studies and mechanism of action [see Clinical Pharmacology (12.1)] , AKEEGA can cause fetal harm and potential loss of pregnancy.
There are no human data on the use of AKEEGA in pregnant women.
Niraparib has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow) [see Warnings and Precautions (5.2)and Nonclinical Toxicology (13.1)] . Due to the potential risk to a fetus based on its mechanism of action, animal developmental and reproductive toxicology studies were not conducted with niraparib.
In animal reproduction studies, oral administration of abiraterone acetate to pregnant rats during organogenesis caused adverse developmental effects at maternal exposures approximately ≥ 0.03 times the human exposure (AUC) at the recommended dose (see Data) .
Data
Animal Data
Niraparib
Niraparib is genotoxic and targets actively dividing cells. Animal developmental and reproductive toxicology studies were not conducted with niraparib.
Abiraterone Acetate
In an embryo-fetal developmental toxicity study in rats, abiraterone acetate caused developmental toxicity when administered at oral doses of 10, 30 or 100 mg/kg/day throughout the period of organogenesis (gestational days 6–17). Findings included embryo-fetal lethality (increased post implantation loss and resorptions and decreased number of live fetuses), fetal developmental delay (skeletal effects) and urogenital effects (bilateral ureter dilation) at doses ≥10 mg/kg/day, decreased fetal ano-genital distance at ≥30 mg/kg/day, and decreased fetal body weight at 100 mg/kg/day. Doses ≥10 mg/kg/day caused maternal toxicity. The doses tested in rats resulted in systemic exposures (AUC) approximately 0.03, 0.1 and 0.3 times, respectively, the AUC in patients receiving 1,000 mg daily of abiraterone acetate.
8.3 Females and Males of Reproductive Potential
Contraception
Males
Based on findings in animal reproduction studies and its mechanism of action, advise males with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of AKEEGA [see Use in Specific Populations (8.1)] .
Infertility
Based on animal studies, AKEEGA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
Safety and effectiveness of AKEEGA in pediatric patients have not been established.
8.5 Geriatric Use
Of the 162 patients with BRCA2gene alteration(s) who received AKEEGA in AMPLITUDE, 40% of patients were less than 65 years, 36% of patients were 65 years to 74 years, and 23% were 75 years and over.
Of the 113 patients with BRCAgene alteration(s) who received AKEEGA in MAGNITUDE, 34.5% of patients were less than 65 years, 38.9% of patients were 65 years to 74 years, and 26.5% were 75 years and over.
No overall differences in effectiveness were observed between patients 65 years of age or older and younger patients in AMPLITUDE or MAGNITUDE. Patients 75 years of age or older who received AKEEGA experienced a higher incidence of fatal adverse reactions than younger patients. The incidence of fatal adverse reactions was 4.3% in patients younger than 75 and 13% in patients 75 or older.
8.6 Hepatic Impairment
Avoid use of AKEEGA in patients with moderate or severe hepatic impairment [see Warnings and Precautions (5.4)and Clinical Pharmacology (12.3)] .
No dosage modification is necessary for patients with mild hepatic impairment.
8.7 Renal Impairment
Monitor patients with severe renal impairment for increased adverse reactions and modify dosage as recommended for adverse reactions [see Clinical Pharmacology (12.3)].
No dosage modification is recommended for patients with mild to moderate renal impairment.
- 10 OVERDOSAGE
-
11 DESCRIPTION
AKEEGA ® (niraparib and abiraterone acetate) tablets contain niraparib tosylate (as the monohydrate) and abiraterone acetate.
Niraparib
Niraparib is a poly (ADP-ribose) polymerase (PARP) inhibitor. The chemical name for niraparib tosylate monohydrate is 2-{4-[(3S)-piperidin-3-yl]phenyl}- 2H-indazole 7-carboxamide 4-methylbenzenesulfonate hydrate (1:1:1). The molecular formula is C 26 H 30 N 4 O 5 S and it has a molecular weight of 510.61 g/mol. The molecular structure is shown below:
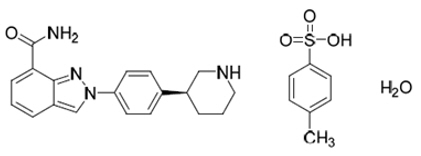
Niraparib tosylate monohydrate is a white to off-white, non-hygroscopic crystalline solid. Niraparib tosylate monohydrate is highly soluble in aqueous media over the pH range 1.2 to 6.8 (1.65–1.77 mg/mL determined at 37 ± 1°C).
Abiraterone Acetate
Abiraterone acetate is the acetyl ester of abiraterone. Abiraterone is an inhibitor of CYP17 (17α-hydroxylase/C17,20-lyase). Its molecular formula is C 26 H 33 N O 2 and it has a molecular weight of 391.55 g/mol. Abiraterone acetate is designated chemically as (3β)-17-(3-pyridinyl) androsta-5,16-dien-3-yl acetate and its structure is:
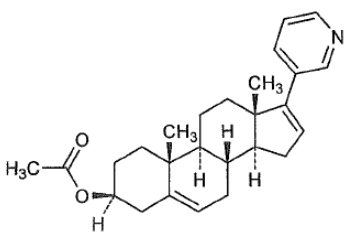
Abiraterone acetate is a white to off-white, non-hygroscopic, crystalline powder. Abiraterone acetate is a lipophilic compound with an octanol-water partition coefficient of 5.12 (Log P) and is practically insoluble in water. The pKa of the aromatic nitrogen is 5.19.
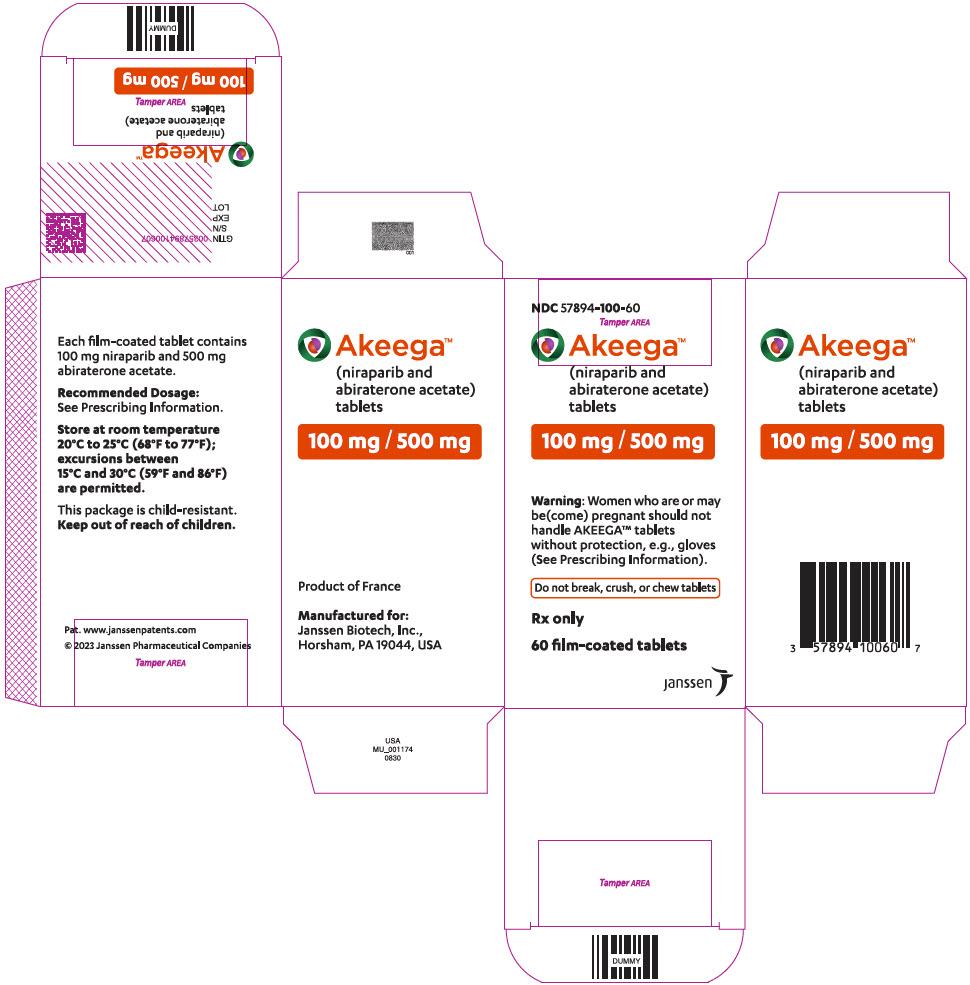
AKEEGA tablets are supplied as 50 mg/500 mg niraparib/abiraterone acetate and 100 mg/500 mg niraparib/abiraterone acetate film-coated tablets for oral administration.
Each AKEEGA tablet (50 mg/500 mg) contains 50 mg of niraparib (equivalent to 76.9 mg niraparib tosylate) and 500 mg of abiraterone acetate.
Each AKEEGA tablet (100 mg/500 mg) contains 100 mg of niraparib (equivalent to 153.7 mg niraparib tosylate) and 500 mg of abiraterone acetate.
AKEEGA tablet core contains the following inactive ingredients: colloidal anhydrous silica, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, silicified microcrystalline cellulose, sodium lauryl sulfate.
- The 50 mg/500 mg tablets are finished with film-coating comprising the following inactive ingredients: iron oxide black, iron oxide red, iron oxide yellow, sodium lauryl sulphate, glycerol monocaprylocaprate, polyvinyl alcohol, talc, and titanium dioxide.
- The 100 mg/500 mg tablets are finished with film-coating comprising the following inactive ingredients: iron oxide red, iron oxide yellow, sodium lauryl sulphate, glycerol monocaprylocaprate, polyvinyl alcohol, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Niraparib is an inhibitor of PARP enzymes, including PARP-1 and PARP-2, that play a role in DNA repair. In vitro studies have shown that niraparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis, and cell death. Increased niraparib‑induced cytotoxicity was observed in tumor cell lines with or without deficiencies in BRCA1/2. Niraparib decreased tumor growth in mouse xenograft models of human cancer cell lines with deficiencies in BRCA1/2 and in human patient‑derived xenograft tumor models with homologous recombination deficiency (HRD) that had either mutated or wild-type BRCA1/2.
Abiraterone acetate is converted in vivo to abiraterone, an androgen biosynthesis inhibitor, that inhibits 17 α-hydroxylase/C17,20-lyase (CYP17). This enzyme is expressed in testicular, adrenal, and prostatic tumor tissues and is required for androgen biosynthesis.
CYP17 catalyzes two sequential reactions: 1) the conversion of pregnenolone and progesterone to their 17α-hydroxy derivatives by 17α-hydroxylase activity and 2) the subsequent formation of dehydroepiandrosterone (DHEA) and androstenedione, respectively, by C17, 20 lyase activity. DHEA and androstenedione are androgens and are precursors of testosterone. Inhibition of CYP17 by abiraterone can also result in increased mineralocorticoid production by the adrenals [see Warnings and Precautions (5.9)] .
Androgen sensitive prostatic carcinoma responds to treatment that decreases androgen levels. Androgen deprivation therapies, such as treatment with GnRH agonists or orchiectomy, decrease androgen production in the testes but do not affect androgen production by the adrenals or in the tumor.
Abiraterone decreased serum testosterone and other androgens in patients in the placebo-controlled clinical trial. It is not necessary to monitor the effect of abiraterone on serum testosterone levels.
Changes in serum prostate specific antigen (PSA) levels may be observed but have not been shown to correlate with clinical benefit in individual patients.
In mouse xenograft models of prostate cancer, the combination of niraparib and abiraterone acetate increased anti-tumor activity when compared to either drug alone.
12.2 Pharmacodynamics
The exposure-response relationship and time-course of pharmacodynamic response for the safety and effectiveness of AKEEGA have not been fully characterized.
Hypertension and Cardiovascular Effects
Niraparib has the potential to cause effects on pulse rate and blood pressure in patients, which may be related to pharmacological inhibition of the dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT) [see Nonclinical Toxicology (13.2)] .
Niraparib increased mean pulse rate by 22.4 to 24.1 beats/min, mean systolic blood pressure by 24.5 mmHg, and mean diastolic pressure by 16.5 mmHg relative to 14.0 to 15.8 beats per min, 18.3 to 19.6 mmHg, and 11.6 mmHg in the placebo arm.
12.3 Pharmacokinetics
Niraparib
Following the administration of AKEEGA, the mean (coefficient of variation [CV%]) C max, ss was 831 ng/mL (32%) and AUC 0–24h, ss was 13,616 ng∙h/mL (36%). The accumulation ratio following daily administration of AKEEGA was 3.5-, and 2.6-fold for niraparib AUC 0–24h and C max.
Niraparib exhibits dose proportional increase in C max and AUC in the dose range of 30 mg (0.15 times the recommended dosage) to 400 mg (2 times the recommended dosage).
Abiraterone Acetate
Following the administration of AKEEGA, the mean (CV%) C max,ss was 151 ng/mL (59%) and AUC 0–24h,ss was 707 ng∙h/mL (59%) for abiraterone. The accumulation ratio following daily administration of AKEEGA was 2-, and 1.8-fold for abiraterone AUC 0–24h and C max.
No major deviation from dose proportionality was observed for abiraterone acetate in the dose range of 250 mg (0.25 times the recommended dosage) to 1,000 mg (the recommended dosage).
Absorption
Niraparib
The median T max was 3 hours after dosing. The absolute bioavailability of niraparib is approximately 73%.
Abiraterone Acetate
The median T max of abiraterone was 1.5 hours after dosing.
Administration of abiraterone acetate with food, compared with administration in a fasted state, results in up to a 10-fold (AUC) and up to a 17-fold (C max) increase in mean systemic exposure of abiraterone, depending on the fat content of the meal. Given the normal variation in the content and composition of meals, taking abiraterone acetate with meals has the potential to result in increased and highly variable exposures.
Distribution
Elimination
Excretion
Specific Populations
No clinically significant effects on the PK of niraparib and abiraterone were observed based on body weight (43.3–165 kg for niraparib and 46–165 kg for abiraterone), age (45–90 years for niraparib and 43–90 years for abiraterone), race/ethnicity (White, Asian, and Hispanic) and mild to moderate renal impairment (CLcr: 30–90 mL/min). Severe renal impairment (CLcr: 15–30 mL/min) has not been studied.
Hepatic Impairment
Niraparib
Mild hepatic impairment did not affect the exposure of niraparib. Moderate hepatic impairment (Total bilirubin > 1.5 to 3 × ULN and any aspartate aminotransferase value) increased niraparib AUC by 56% compared to that of patients with normal hepatic function.
Abiraterone Acetate
Mild (Child-Pugh score of 5 to 6; Child-Turcotte-Pugh Class A) hepatic impairment increased abiraterone (AUC) by 1.1-fold and moderate (Child-Pugh score of 7 to 9; Child-Turcotte-Pugh Class B) hepatic impairment increased abiraterone (AUC) by 3.6-fold compared to subjects with normal hepatic function.
Severe (Child-Pugh score of 10 to 15; Child-Turcotte-Pugh Class C) hepatic impairment increased abiraterone AUC by 7-fold and the fraction of free drug increased by 2-fold in subjects compared to subjects with normal hepatic function.
Drug Interactions Studies
Niraparib
In Vitro Studies
- Inhibition of Cytochrome P450 ( CYP) Enzymes: Niraparib is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4.
- Induction of CYP Enzymes:Niraparib is not a CYP3A4 inducer. Niraparib induces CYP1A2 in vitro.
- Inhibition of Uridine 5'-Diphospho-Glucuronosyltransferases (UGTs): Niraparib did not inhibit UGT1A1, UGT1A4, UGT1A9, and UGT2B7.
-
Inhibition of Transporter Systems: Niraparib inhibits BCRP, but does not inhibit P-gp, BSEP, or MRP2.
Niraparib inhibits MATE 1 and 2. - Substrate of Transporter Systems: Niraparib is a substrate of P-gp and BCRP. Niraparib is not a substrate of BSEP, MRP2, or MATE1 or 2.
Abiraterone Acetate
Clinical Studies
- Effect of Strong CYP3A4 Inducers on Abiraterone: Coadministration of rifampin (strong CYP3A4 inhibitor) decreased abiraterone mean AUC by 55%.
- Effect of Strong CYP3A4 Inhibitors on Abiraterone: Coadministration of ketoconazole (strong CYP3A4 inhibitor) had no clinically meaningful effect on the pharmacokinetics of abiraterone.
- Effect of Abiraterone Acetate on CYP2D6 Substrates: The C max and AUC of dextromethorphan (CYP2D6 substrate) were increased 2.8- and 2.9-fold, respectively when dextromethorphan 30 mg was given with abiraterone acetate 1,000 mg daily (plus prednisone). The AUC for dextrorphan, the active metabolite of dextromethorphan, increased approximately 1.3-fold.
- Effect of Abiraterone Acetate on CYP1A2 Substrates: When abiraterone acetate (plus prednisone) was given with a single dose of 100 mg theophylline (CYP1A2 substrate), no increase in systemic exposure of theophylline was observed.
- Effect of Abiraterone Acetate on CYP2C8 Substrates: The AUC of pioglitazone (CYP2C8 substrate) was increased by 46% when pioglitazone was given to healthy subjects with a single dose of abiraterone acetate.
In vitro Studies
- Cytochrome P450 (CYP) Enzymes: Abiraterone is a substrate of CYP3A4 and has the potential to inhibit CYP1A2, CYP2D6, CYP2C8 and to a lesser extent CYP2C9, CYP2C19 and CYP3A4/5.
- Transporter Systems: Abiraterone acetate and abiraterone are not substrates of P-gp. Abiraterone acetate is an inhibitor of P-gp. Abiraterone and its major metabolites were inhibitors of OATP1B1.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Niraparib
Carcinogenicity studies have not been conducted with niraparib.
Niraparib was clastogenic in an in vitro mammalian chromosomal aberration assay and in an in vivo rat bone marrow micronucleus assay. This clastogenicity is consistent with genomic instability resulting from the primary pharmacology of niraparib and indicates potential for genotoxicity in humans. Niraparib was not mutagenic in a bacterial reverse mutation assay (Ames) test.
Fertility studies in animals have not been conducted with niraparib. In repeat-dose oral toxicity studies, niraparib was administered daily for up to 3 months' duration in rats and dogs. Reduced sperm, spermatids, and germ cells in epididymides and testes were observed at doses ≥10 mg/kg and ≥1.5 mg/kg in rats and dogs, respectively. These dose levels resulted in systemic exposures approximately 0.5 and 0.02 times, respectively, the human exposure (AUC 0–24h) at the dose of 200 mg daily. There was a trend toward reversibility of these findings 4 weeks after dosing was stopped.
Abiraterone Acetate
A two-year carcinogenicity study was conducted in rats at oral abiraterone acetate doses of 5, 15, and 50 mg/kg/day for males and 15, 50, and 150 mg/kg/day for females. Abiraterone acetate increased the combined incidence of interstitial cell adenomas and carcinomas in the testes at all dose levels tested. This finding is considered to be related to the pharmacological activity of abiraterone. Rats are regarded as more sensitive than humans to developing interstitial cell tumors in the testes. Abiraterone acetate was not carcinogenic in female rats at exposure levels up to 0.8 times the human clinical exposure (1,000 mg daily) based on AUC. Abiraterone acetate was not carcinogenic in a 6-month study in the transgenic (Tg.rasH2) mouse.
Abiraterone acetate and abiraterone were not mutagenic in an in vitro microbial mutagenesis (Ames) assay or clastogenic in an in vitro cytogenetic assay using primary human lymphocytes or an in vivo rat micronucleus assay.
In repeat-dose toxicity studies in male rats (13- and 26-weeks) and monkeys (39-weeks), atrophy, aspermia/hypospermia, and hyperplasia in the reproductive system were observed at ≥50 mg/kg/day in rats and ≥250 mg/kg/day in monkeys and were consistent with the antiandrogenic pharmacological activity of abiraterone. These effects were observed in rats at systemic exposures similar to humans and in monkeys at exposures approximately 0.6 times the AUC in humans at 1,000 mg daily.
In a fertility study in male rats, reduced organ weights of the reproductive system, sperm counts, sperm motility, altered sperm morphology and decreased fertility were observed in animals dosed for 4 weeks at ≥30 mg/kg/day orally. Mating of untreated females with males that received 30 mg/kg/day oral abiraterone acetate resulted in a reduced number of corpora lutea, implantations and live embryos and an increased incidence of pre-implantation loss. Effects on male rats were reversible after 16 weeks from the last abiraterone acetate administration.
In a fertility study in female rats, animals dosed orally for 2 weeks until day 7 of pregnancy at ≥30 mg/kg/day had an increased incidence of irregular or extended estrous cycles and pre-implantation loss (300 mg/kg/day). There were no differences in mating, fertility, and litter parameters in female rats that received abiraterone acetate. Effects on female rats were reversible after 4 weeks from the last abiraterone acetate administration.
The dose of 30 mg/kg/day in rats is approximately 0.3 times the recommended dose of 1,000 mg/day based on body surface area.
In 13- and 26-week studies in rats and 13- and 39-week studies in monkeys, a reduction in circulating testosterone levels occurred with abiraterone acetate at approximately one half the human clinical exposure based on AUC. As a result, decreases in organ weights and toxicities were observed in the male and female reproductive system, adrenal glands, liver, pituitary (rats only), and male mammary glands. The changes in the reproductive organs are consistent with the antiandrogenic pharmacological activity of abiraterone acetate.
13.2 Animal Toxicology and/or Pharmacology
Niraparib
In vitro, niraparib bound to DAT, NET, and SERT and inhibited uptake of norepinephrine and dopamine in cells with IC 50 values that were lower than the C min at steady-state in patients receiving the 200 mg dose. Niraparib has the potential to cause effects in patients related to inhibition of these transporters (e.g., cardiovascular, central nervous system). Intravenous administration of niraparib to vagotomized dogs over 30 minutes at 1, 3, and 10 mg/kg resulted in an increased range of arterial pressures of 13% to 20%, 18% to 27%, and 19% to 25%, respectively, and increased range of heart rates of 2% to 11%, 4% to 17%, and 12% to 21%, respectively, above pre-dose levels. The unbound plasma concentrations of niraparib in dogs at these dose levels were approximately 1.2, 3.9, and 15.5 times the unbound C max at steady state in patients receiving the 200 mg dose.
In addition, niraparib crossed the blood-brain barrier in rats and monkeys following oral administration. The cerebrospinal fluid plasma C max ratios of niraparib administered at 10 mg/kg orally to two rhesus monkeys were 0.10 and 0.52.
Abiraterone Acetate
A dose-dependent increase in cataracts was observed in rats after daily oral abiraterone acetate administration for 26 weeks starting at ≥50 mg/kg/day (similar to the human clinical exposure (AUC) at 1,000 mg dose daily). In a 39-week monkey study with daily oral abiraterone acetate administration, no cataracts were observed at higher doses (2 times greater than the clinical exposure (AUC) at 1,000 mg dose daily).
-
14 CLINICAL STUDIES
14.1 BRCA2-mutated Metastatic Castration-Sensitive Prostate Cancer (mCSPC)
The efficacy of AKEEGA was investigated in AMPLITUDE (NCT04497844), a randomized double-blind, placebo-controlled, multi-cohort, multi-center study in which 696 patients with homologous recombination repair (HRR) gene-mutated (HRRm) mCSPC were randomized (1:1) to receive niraparib 200 mg and abiraterone acetate 1,000 mg (N=348) or placebo and abiraterone acetate (N=348). All patients received prednisone 5 mg daily and were required to have androgen deprivation therapy (ADT) (medical or surgical) >14 days prior to randomization. The only allowable prior systemic therapy in the mCSPC setting, was up to 45 days of abiraterone acetate, up to 6 cycles of docetaxel, and up to 6 months of ADT.
Randomization was stratified by HRR gene alteration ( BRCA2versus CDK12versus all other pathogenic alterations), prior docetaxel use (yes versus no), and volume of disease at screening (high versus low).
Of the 696 patients enrolled, 323 were randomized as having BRCA2gene mutation ( BRCA2m). Mutation status was determined prospectively using the Foundation One CDx tissue assay or other clinical trial assays.
Among the 323 patients with BRCA2m the median age was 66 years (range 41; 92); 68% were White, 25% Asian, 4% Black, and 3% other or not reported; 10% were Hispanic or Latino; and baseline ECOG performance status was 0 (68%), 1 (30%) or 2 (1.2%). 16% had received prior docetaxel and 11% received prior abiraterone acetate for up to 45 days for mCSPC. 40% had bone-only metastases and 15% had visceral metastases, 10% had BRCA2 mutations in combination with mutations in other HRR genes.
The major efficacy outcome measure was radiographic progression free survival (rPFS) determined by investigator-assessed radiographic progression by bone scan (according to PCWG3 criteria) or soft tissue lesions by CT or MRI (according to RECIST 1.1 criteria) or death, whichever occurred first. Overall Survival (OS) and Time to Symptomatic Progression (TSP) were additional efficacy outcome measures. A statistically significant improvement in rPFS for niraparib and abiraterone acetate compared to placebo and abiraterone acetate was observed in the overall population of patients with HRRm. In an exploratory analysis in the subgroup of 373 patients with non- BRCA2mutations, the investigator-assessed rPFS hazard ratio was 0.88 (95% CI: 0.63, 1.24), indicating that the improvement in the overall population was primarily attributed to the results seen in the subgroup of patients with BRCA2mutation.
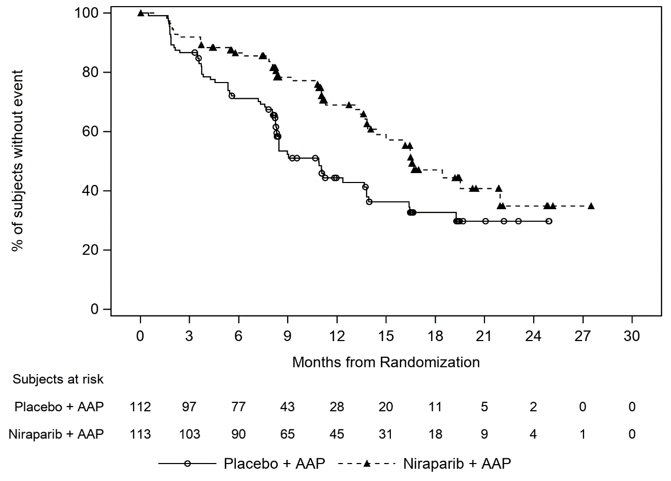
The efficacy results are presented in Table 6 and Figure 1 for patients with BRCA2mutations in AMPLITUDE.
Table 6: Efficacy Results from the BRCA2m Subgroup of the AMPLITUDE Study Endpoints AKEEGA
(N=162)Placebo +Abiraterone Acetate
(N=161)NE = not estimable - * Investigator-assessed
- † Calculated using an unstratified Cox proportional hazards model
Radiographic Progression-free Survival * Events 48 (30%) 82(51%) Median (95% CI) time to event (months) NE (41, NE) 26 (18, 28) Hazard Ratio (95% CI) † 0.46 (0.32, 0.66) At the first interim analysis for OS, 91 deaths occurred in the BRCA2m population, 36 [22%] in the AKEEGA arm) and 55 [34%] in the placebo and abiraterone acetate arm.
Figure 1: Kaplan-Meier Plot of Radiographic Progression-Free Survival in the BRCA2m Population (AMPLITUDE)
Treatment with AKEEGA resulted in a delay in TSP (HR = 0.41, 95% CI= 0.26, 0.65). TSP was defined as the time from randomization to the time of symptomatic progression, which included use of external beam radiation for skeletal or pelvic symptoms, cancer-related morbid events, initiation of new systemic anti-cancer therapy, and other cancer-related procedures.
14.2 BRCA-mutated Metastatic Castration-Resistant Prostate Cancer (mCRPC)
The efficacy of AKEEGA was investigated in Cohort 1 of MAGNITUDE (NCT03748641), a randomized double-blind, placebo-controlled, multi-cohort, multi-center study in which 423 patients with homologous recombination repair (HRR) gene-mutated (HRRm) mCRPC were randomized (1:1) to receive niraparib 200 mg and abiraterone 1,000 mg (N=212) or placebo and abiraterone (N=211) until unacceptable toxicity or progression. All patients received prednisone 10 mg daily and a GnRH analog or had prior bilateral orchiectomy. Patients with mCRPC who had not received prior systemic therapy in the mCRPC setting except for a short duration of prior abiraterone acetate plus prednisone (up to four months) and ongoing ADT, were eligible. Patients could have received prior docetaxel or androgen-receptor (AR) targeted therapies in either the metastatic castration-sensitive prostate cancer (mCSPC) or non-metastatic castration-resistant prostate cancer (nmCRPC) setting.
Randomization was stratified by prior docetaxel for mCSPC (yes or no), prior AR targeted therapy for mCSPC or nmCRPC (yes or no), prior abiraterone acetate for mCRPC (yes or no), and BRCA-status ( BRCAm vs. other).
Of the 423 patients enrolled, 225 (53%) had BRCA gene mutations ( BRCA m). Mutation status of BRCA genes was determined prospectively using the Foundation One CDx tissue assay or other clinical trial assays.
Among the 225 patients with BRCA m, the median age was 68 years (range 43–100) and 66% were 65 years of age or older; 72% were White, 17% Asian, and 1% Black, and 10% other or not reported; 12% were Hispanic or Latino; and baseline ECOG performance status (PS) was 0 (66%) or 1 (34%). Twenty-four percent had received prior docetaxel, 5% received prior AR-targeted therapy for mCSPC or nmCRPC, and 26% received prior abiraterone acetate plus prednisone for up to 4 months for mCRPC. Thirty-seven percent had bone-only metastases and 21% had visceral metastases. Seven percent had BRCA1 mutations, 78% had BRCA2 mutations, and 15% had BRCA mutations in combination with mutations in other HRR genes.
The major efficacy outcome measure was radiographic progression free survival (rPFS) determined by blinded independent central radiology (BICR) review evaluated per Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 (soft tissue lesions) and Prostate Cancer Working Group-3 (PCWG-3) criteria (bone lesions). Overall survival (OS) was an additional efficacy outcome measure.
A statistically significant improvement in rPFS for niraparib plus abiraterone compared to placebo plus abiraterone was observed in BRCA m patients, and the Cohort 1 intention to treat (ITT) population. In an exploratory analysis in the subgroup of 198 (47%) patients with non- BRCA mutations, the rPFS hazard ratio was 0.99 (95% CI: 0.67, 1.44) and the OS hazard ratio was 1.13 (95% CI: 0.77, 1.64), indicating that the improvement in the ITT population was primarily attributed to the results seen in the subgroup of patients with BRCA m.
The efficacy results are presented in Table 7 and Figures 2 and 3 for patients in Cohort 1 with BRCA mutations.
Table 7: Efficacy Results from the BRCAm Subgroup of the MAGNITUDE Study Endpoints AKEEGA
(N=113)Placebo + Abiraterone Acetate
(N=112)NE = not estimable - * rPFS results based on blinded independent central review at primary analysis.
- † Cox proportional hazards model stratified by prior docetaxel (yes vs. no) and prior abiraterone (yes vs. no).
- ‡ Stratified log-rank test two-sided p-value
Radiographic Progression-free Survival * Event of disease progression or death (%) 45 (40%) 64 (57%) Median, months (95% CI) 16.6 (13.9, NE) 10.9 (8.3, 13.8) Hazard Ratio †(95% CI) 0.53 (0.36, 0.79) p-value ‡ 0.0014 At the protocol pre-specified final OS analysis in Cohort 1, 60 (53%) deaths and 70 (63%) deaths were observed in the AKEEGA arm and placebo arm, respectively, for patients with BRCAm. In an exploratory OS analysis in the subgroup of patients with BRCAm, the median in the AKEEGA arm was 30.4 (95% CI: 27.6, NE) and 28.6 months (95% CI: 23.8, 33.0) in the placebo arm, with an OS hazard ratio of 0.79 (95% CI: 0.55, 1.12).
Figure 2: Kaplan-Meier Plot of BICR Assessed Radiographic Progression-Free Survival in the BRCAm Population (MAGNITUDE, primary analysis)
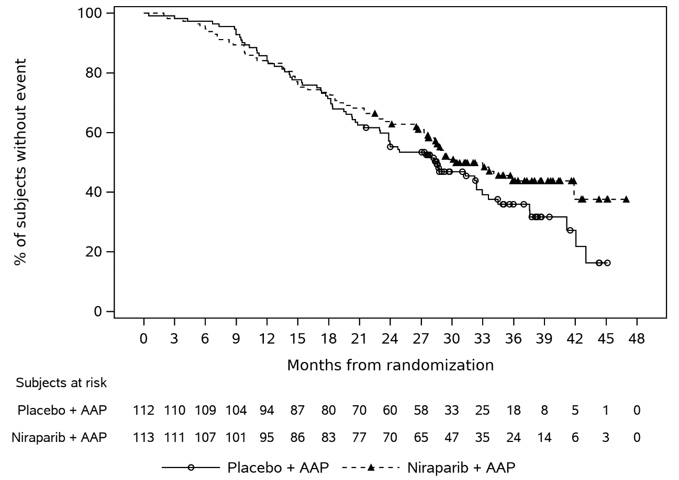
Figure 3: Kaplan-Meier Plot of Overall Survival in the BRCAm Population (MAGNITUDE, final analysis)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
AKEEGA ® (niraparib and abiraterone acetate) tablets are available in the strengths and packages listed below:
- AKEEGA 50 mg/500 mg film-coated tablets
Yellowish orange to yellowish brown, oval, film-coated tablets debossed with "N 50 A" on one side and plain on the other side. They are available in bottles of 60 tablets.
NDC: 57894-050-60 - AKEEGA 100 mg/500 mg film-coated tablets
Orange, oval, film-coated tablets debossed with "N 100 A" on one side and plain on the other side. They are available in bottles of 60 tablets.
NDC: 57894-100-60
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Based on its mechanism of action, AKEEGA may harm a developing fetus. Females who are or may become pregnant should handle AKEEGA tablets with protection, e.g., gloves [see Use in Specific Populations (8.1)] .
- AKEEGA 50 mg/500 mg film-coated tablets
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hematologic Adverse Reactions
- Advise patients that periodic monitoring of their blood counts is recommended. Advise patients to contact their healthcare provider for new onset of pallor, weakness, dyspnea, fatigue, bleeding, fever, or symptoms of infection [see Warnings and Precautions (5.1, 5.2)] .
Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions
- Inform patients that AKEEGA is associated with, hypokalemia that may lead to QT prolongation. Advise patients that hypertension, hypokalemia, and fluid retention will be monitored at least weekly for the first two months, then once a month. Advise patients to adhere to corticosteroids and to report symptoms of hypokalemia or edema to their healthcare provider [see Warnings and Precautions (5.3)] .
Hepatotoxicity and Hepatic Impairment
- Inform patients that AKEEGA is associated with severe hepatotoxicity. Inform patients that their liver function will be monitored using blood tests. Advise patients to immediately report symptoms of hepatotoxicity to their healthcare provider [see Warnings and Precautions (5.4)] .
Adrenocortical Insufficiency
- Inform patients that AKEEGA with prednisone is associated with adrenal insufficiency. Advise patients to report symptoms of adrenocortical insufficiency to their healthcare provider [see Warnings and Precautions (5.5)] .
Hypoglycemia
- Inform patients that AKEEGA is associated with hypoglycemia. Advise patients with diabetes to monitor blood glucose during and after discontinuation of treatment with AKEEGA [see Warnings and Precautions (5.6)] .
Posterior Reversible Encephalopathy Syndrome
- Inform patients that they are at risk of developing posterior reversible encephalopathy syndrome (PRES) that can present with signs and symptoms including seizure, headaches, altered mental status, or vision changes. Advise patients to contact their healthcare provider if they develop any of these signs or symptoms [see Warnings and Precautions (5.8)] .
Dosage and Administration
- Inform patients that AKEEGA is taken orally once daily with prednisone daily (according to their healthcare provider's instructions) and to not interrupt or stop either of these medications without consulting their healthcare provider [see Dosage and Administration (2.2)] .
- Inform patients coadministered a gonadotropin-releasing hormone (GnRH) analog therapy that they need to maintain this treatment during the course of treatment with AKEEGA [see Dosage and Administration (2.2)] .
- Inform patients that in the event of a missed daily dose of AKEEGA, they should take their normal dose as soon as possible on the same day and resume their next dose at the normal schedule on the following day. The patient should not take extra tablets to make up the missed dose [see Dosage and Administration (2.2)] .
- Instruct patients to take AKEEGA tablets as a single dose once daily on an empty stomach. Instruct patients to take AKEEGA on an empty stomach at least one hour before or two hours after food. AKEEGA taken with food causes increased exposure and may result in adverse reactions. Instruct patients to swallow tablets whole with water and not to break, crush, or chew the tablets [see Dosage and Administration (2.2)] .
Embryo-Fetal Toxicity
- Inform patients that AKEEGA may harm a developing fetus and can cause loss of pregnancy [see Warnings and Precautions (5.9)and Use in Specific Populations (8.1)] .
- Advise males with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of AKEEGA [see Use in Specific Populations (8.3)] .
- Advise females who are pregnant or may become pregnant to handle AKEEGA tablets with protection, e.g., gloves [see Use in Specific Populations (8.1)and How Supplied/Storage and Handling (16)] .
Infertility
- Advise male patients that AKEEGA may impair fertility [see Use in Specific Populations (8.3)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 12/2025 PATIENT INFORMATION
AKEEGA ® (a kee' gah)
(niraparib and abiraterone acetate)
tabletsWhat is the most important information I should know about AKEEGA?
AKEEGA may cause serious side effects including:-
Bone marrow problems called myelodysplastic syndrome (MDS) or a type of cancer of the blood called acute myeloid leukemia (AML).MDS or AML that may lead to death has happened in people treated with AKEEGA. If you develop MDS or AML, your healthcare provider will stop treatment with AKEEGA.
Symptoms of low blood cell counts (low red blood cells, low white blood cells, and low platelets) are common during treatment with AKEEGA but can also be a sign of serious bone marrow problems, including MDS and AML. Tell your healthcare provider if you have any of the following symptoms during treatment with AKEEGA:
- pale skin
- weakness
- shortness of breath
- feeling tired
- bruising or bleeding more easily
- fever
- frequent infections
- blood in urine or stool
- weight loss
Your healthcare provider will do blood tests to check your blood cell counts: - weekly during the first month of treatment,
- every 2 weeks for the next 2 months of treatment,
- monthly for the remainder of the year,
- then every other month and as needed during treatment with AKEEGA.
See " What are the possible side effects of AKEEGA?" for more information about side effects. What is AKEEGA?
AKEEGA is a prescription medicine used with prednisone to treat adults with prostate cancer:- who have a certain type of abnormal BRCA gene, and
- whose prostate cancer has spread to other parts of the body (metastatic prostate cancer).
It is not known if AKEEGA is safe and effective in females.
It is not known if AKEEGA is safe and effective in children.Before taking AKEEGA, tell your healthcare provider about all of your medical conditions, including if you: - have high blood pressure or heart problems
- have low blood potassium levels
- have liver or kidney problems
- have a history of adrenal problems
- have diabetes
- are receiving any other treatment for prostate cancer
- are pregnant or plan to become pregnant. AKEEGA can cause harm to your unborn baby and loss of pregnancy (miscarriage). Females who are or may become pregnant should handle AKEEGA tablets with protection, such as gloves.
- have a partner who is pregnant or may become pregnant.
- Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment and for 4 months after the last dose of AKEEGA.
- are breastfeeding or plan to breastfeed. It is not known if AKEEGA passes into your breastmilk.
How should I take AKEEGA? - Take AKEEGA and prednisone exactly as your healthcare provider tells you.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with AKEEGA if you have certain side effects.
- Do not change or stop taking your prescribed dose of AKEEGA or prednisone without talking with your healthcare provider first.
- Take your prescribed dose of AKEEGA 1 time a day.
- Take AKEEGA on an empty stomach at least 1 hour before or 2 hours after food. Taking AKEEGA with food may cause more of the medicine to be absorbed by the body than is needed and this may cause side effects.
- Swallow AKEEGA tablets whole with water. Do not break, crush, or chew tablets.
- If you miss a dose of AKEEGA, take the dose as soon as possible on the same day. Return to your normal schedule on the following day. Do not take extra tablets to make up the missed dose.
- You should start or continue a gonadotropin-releasing hormone (GnRH) analog therapy during your treatment with AKEEGA unless you have had a surgery to lower the amount of testosterone in your body (surgical castration).
- If you take too much AKEEGA, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of AKEEGA?
AKEEGA may cause serious side effects, including:- See " What is the most important information I should know about AKEEGA?"
- Low blood potassium levels (hypokalemia), fluid retention (edema), high blood pressure (hypertension) and heart problems. To decrease the chance of this happening, you must take prednisone with AKEEGA exactly as your healthcare provider tells you. Your healthcare provider will check your blood pressure, do blood tests to check your potassium levels, and check for any signs and symptoms of fluid retention at least weekly for the first 2 months of treatment, then 1 time a month during treatment with AKEEGA. Tell your healthcare provider if you have any of the following symptoms:
- dizziness
- fast or irregular heartbeats
- feel faint or lightheaded
- headache
- confusion
- muscle weakness
- pain in your legs
- swelling in your hands, ankles, legs or feet
- Liver problems. Severe liver problems, liver failure and death has happened in people treated with abiraterone acetate, one of the medicines in AKEEGA. Your healthcare provider will do blood tests to check your liver function before starting treatment with AKEEGA, every 2 weeks for the first 3 months of treatment, and then monthly thereafter during treatment with AKEEGA. Tell your healthcare provider right away if you develop any symptoms of liver problems, including:
- yellowing of the skin or eyes
- darkening of the urine
- severe nausea or vomiting
- Adrenal problems. Adrenal problems may happen if you stop taking prednisone, get an infection, or are under stress. Tell your healthcare provider right away if you develop any symptoms of adrenal problems, including:
- feeling tired
- weakness
- feeling dizzy or lightheaded
- nausea or vomiting
- weight loss
- Low blood sugar (hypoglycemia).AKEEGA may cause low blood sugar in people taking medicines for diabetes. Severe low blood sugar has happened in people who take certain medicines for diabetes and were treated with abiraterone acetate, one of the medicines in AKEEGA. You and your healthcare provider should check your blood sugar levels during treatment and after you stop treatment with AKEEGA. Your healthcare provider may need to change the dose of your diabetes medicine to decrease your risk of low blood sugar. Tell your healthcare provider right away if you have any of the following signs or symptoms of low blood sugar, including:
- headache
- drowsiness
- weakness
- dizziness
- confusion
- irritability
- hunger
- fast heartbeat
- sweating
- feeling jittery
- Increased risk of bone fracture and death when abiraterone acetate, one of the medicines in AKEEGA, and prednisone or prednisolone is used in combination with a type of radiation called Radium 223 (Ra-223) dichloride. You should not receive treatment with Ra-223 dichloride for at least 5 days after your last dose of AKEEGA with prednisone. Tell your healthcare provider about any other treatments you are taking for prostate cancer.
- Posterior Reversible Encephalopathy Syndrome (PRES). PRES is a condition that affects the brain and may happen during treatment with AKEEGA. If you have headache, vision changes, confusion, or seizure with or without high blood pressure, please contact your healthcare provider.
- decreased hemoglobin
- decreased lymphocytes
- muscle and bone pain
- tiredness
- decreased platelets
- changes in liver function blood tests
- constipation
- high blood pressure
- nausea
- decreased neutrophils
- changes in kidney function blood tests
- increased potassium level in the blood
- decreased potassium level in the blood
- swelling in your legs or feet
- increased bilirubin levels in the blood
- respiratory tract infection
- irregular heartbeat (arrhythmia)
AKEEGA may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of AKEEGA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store AKEEGA? - Store AKEEGA at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of AKEEGA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AKEEGA for a condition for which it was not prescribed. Do not give AKEEGA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about AKEEGA that is written for health professionals.What are the ingredients in AKEEGA?
Active ingredients: niraparib tosylate and abiraterone acetate
Inactive ingredients:
Core tablet: colloidal anhydrous silica, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, silicified microcrystalline cellulose, sodium lauryl sulfate.
50 mg/500 mg film-coated tablets: The film-coating contains iron oxide black, iron oxide red, iron oxide yellow, sodium lauryl sulphate, glycerol monocaprylocaprate, polyvinyl alcohol, talc, and titanium dioxide.
100 mg/500 mg film-coated tablets: The film-coating contains iron oxide red, iron oxide yellow, sodium lauryl sulphate, glycerol monocaprylocaprate, polyvinyl alcohol, talc, and titanium dioxide.
Manufactured for: Janssen Biotech, Inc., Horsham, PA 19044, USA
For patent information: www.janssenpatents.com
© Johnson & Johnson and its affiliates 2025
For more information, call Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or go to www.akeegahcp.com. -
Bone marrow problems called myelodysplastic syndrome (MDS) or a type of cancer of the blood called acute myeloid leukemia (AML).MDS or AML that may lead to death has happened in people treated with AKEEGA. If you develop MDS or AML, your healthcare provider will stop treatment with AKEEGA.
-
PRINCIPAL DISPLAY PANEL - 100 mg / 500 mg Bottle Carton
NDC: 57894-100-60
Tamper AREA
Akeega™
(niraparib and
abiraterone acetate)
tablets100 mg / 500 mg
Warning: Women who are or may
be(come) pregnant should not
handle AKEEGA™ tablets
without protection, e.g., gloves
(See Prescribing Information).Do not break, crush, or chew tablets
Rx only
60 film-coated tablets
janssen
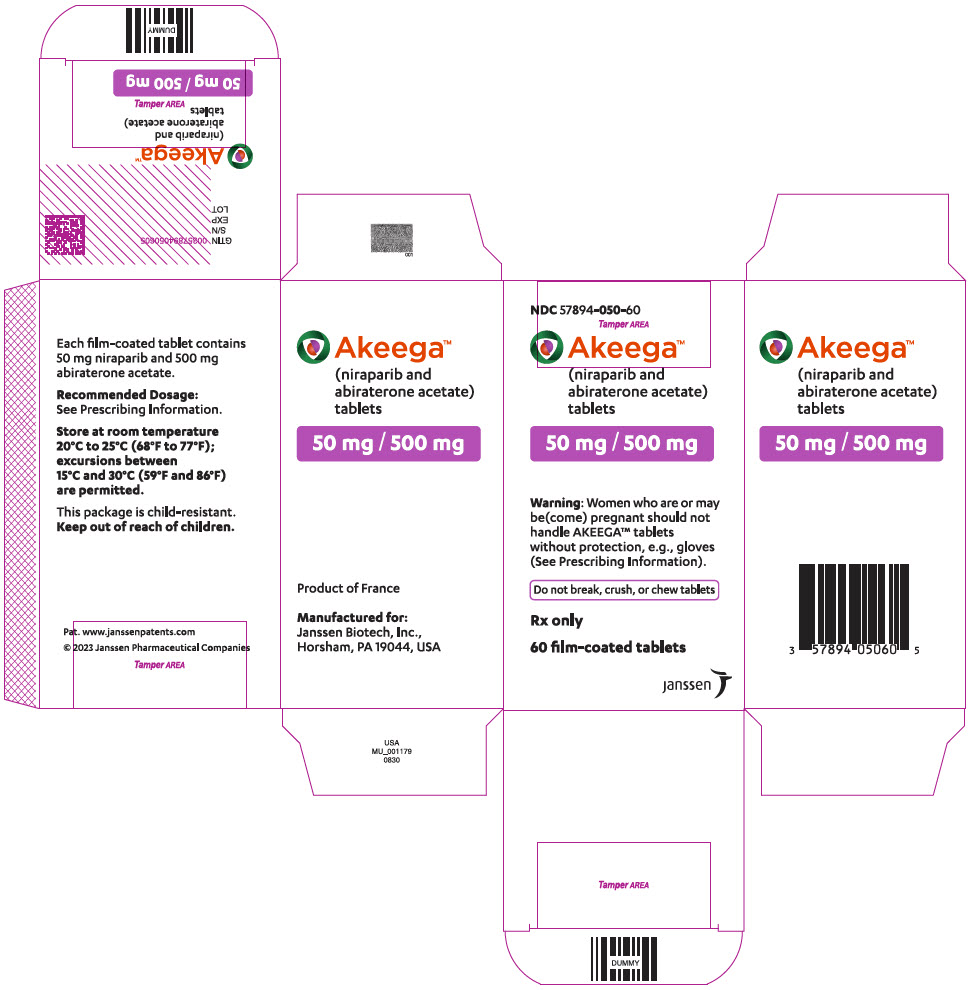
-
PRINCIPAL DISPLAY PANEL - 50 mg / 500 mg Bottle Carton
NDC: 57894-050-60
Tamper AREA
Akeega™
(niraparib and
abiraterone acetate)
tablets50 mg / 500 mg
Warning: Women who are or may
be(come) pregnant should not
handle AKEEGA™ tablets
without protection, e.g., gloves
(See Prescribing Information).Do not break, crush, or chew tablets
Rx only
60 film-coated tablets
janssen
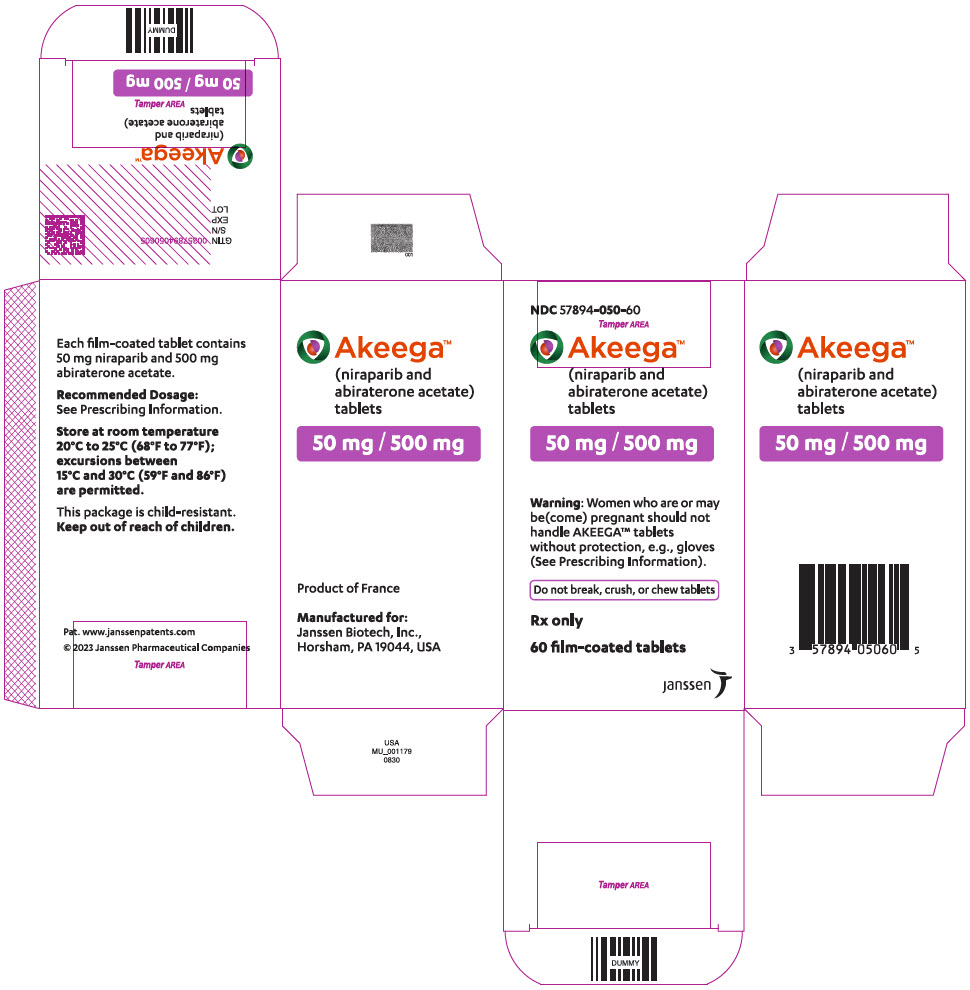
-
INGREDIENTS AND APPEARANCE
AKEEGA
niraparib tosylate monohydrate and abiraterone acetate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57894-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIRAPARIB TOSYLATE MONOHYDRATE (UNII: 195Q483UZD) (NIRAPARIB - UNII:HMC2H89N35) NIRAPARIB 100 mg ABIRATERONE ACETATE (UNII: EM5OCB9YJ6) (ABIRATERONE - UNII:G819A456D0) ABIRATERONE ACETATE 500 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown (yellow to yellowish-brown) Score no score Shape OVAL (capsule-shaped) Size 22mm Flavor Imprint Code N;100;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57894-100-60 1 in 1 CARTON 08/11/2023 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216793 08/11/2023 AKEEGA
niraparib tosylate monohydrate and abiraterone acetate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57894-050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIRAPARIB TOSYLATE MONOHYDRATE (UNII: 195Q483UZD) (NIRAPARIB - UNII:HMC2H89N35) NIRAPARIB 50 mg ABIRATERONE ACETATE (UNII: EM5OCB9YJ6) (ABIRATERONE - UNII:G819A456D0) ABIRATERONE ACETATE 500 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown (yellow to yellowish-brown) Score no score Shape OVAL (capsule-shaped) Size 22mm Flavor Imprint Code N;50;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57894-050-60 1 in 1 CARTON 08/11/2023 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216793 08/11/2023 Labeler - Janssen Biotech, Inc. (099091753) Establishment Name Address ID/FEI Business Operations Shanghai SynTheAll Pharmaceutical Co., Ltd. 545342792 api manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Changzhou SynTheAll Pharmaceutical Co., Ltd. 544385021 api manufacture(57894-100, 57894-050) , manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Jiangsu Jiaerke Pharmaceuticals Group Corp., Ltd. 527224249 api manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Zhejiang Xianju Junye Pharmaceutical Co., Ltd. 421340422 api manufacture(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Pharmacia & Upjohn Company LLC 618054084 manufacture(57894-100, 57894-050) , pack(57894-100, 57894-050) , label(57894-100, 57894-050) , analysis(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutica NV 400345889 api manufacture(57894-100, 57894-050) , manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Patheon France S.A.S 543127229 analysis(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Janssen Cilag SpA 542797928 pack(57894-100, 57894-050) Establishment Name Address ID/FEI Business Operations Johnson & Johnson Private Limited 677603030 analysis(57894-100, 57894-050)
Trademark Results [AKEEGA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AKEEGA 90714758 not registered Live/Pending |
JOHNSON & JOHNSON 2021-05-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.