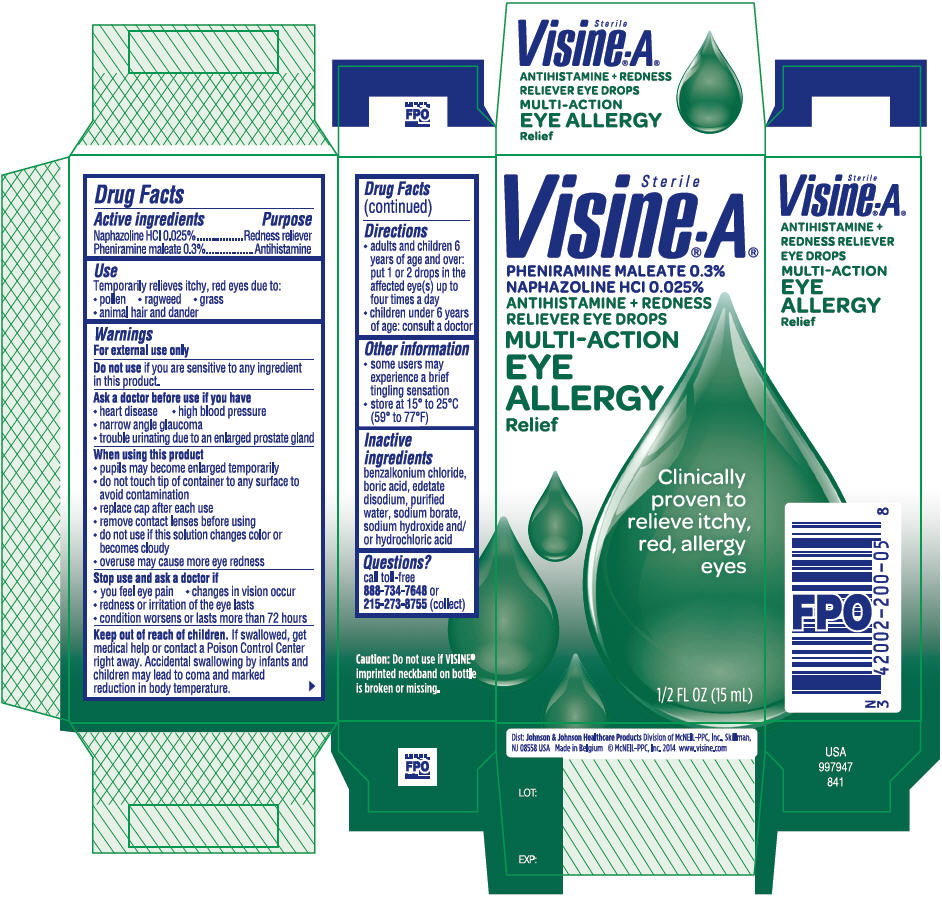
Visine®-A® MULTI-ACTION EYE ALLERGY Relief
Visine A by
Drug Labeling and Warnings
Visine A by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VISINE A MULTI-ACTION EYE ALLERGY RELIEF- naphazoline hydrochloride and pheniramine maleate solution/ drops
Johnson & Johnson Consumer Inc.
----------
Visine®-A® MULTI-ACTION EYE ALLERGY Relief
Warnings
For external use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- narrow angle glaucoma
- trouble urinating due to an enlarged prostate gland
When using this product
- pupils may become enlarged temporarily
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- overuse may cause more eye redness
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to four times a day
- children under 6 years of age: consult a doctor
Other information
- some users may experience a brief tingling sensation
- store at 15° to 25°C (59° to 77°F)
| VISINE A
MULTI-ACTION EYE ALLERGY RELIEF
naphazoline hydrochloride and pheniramine maleate solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 1/2020
Document Id: 08a0c95d-8192-4524-b3ec-89f4773702de
Set id: 82500e4c-bc01-4ce0-80d8-6e9f0be99163
Version: 7
Effective Time: 20200103
Johnson & Johnson Consumer Inc.