ACL11KC NERVE BLOCK- kit
ACL11KC NERVE BLOCK by
Drug Labeling and Warnings
ACL11KC NERVE BLOCK by is a Other medication manufactured, distributed, or labeled by Clint Pharmaceuticals, Inc., Smiths Medical ASD, Inc., Aplicare, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
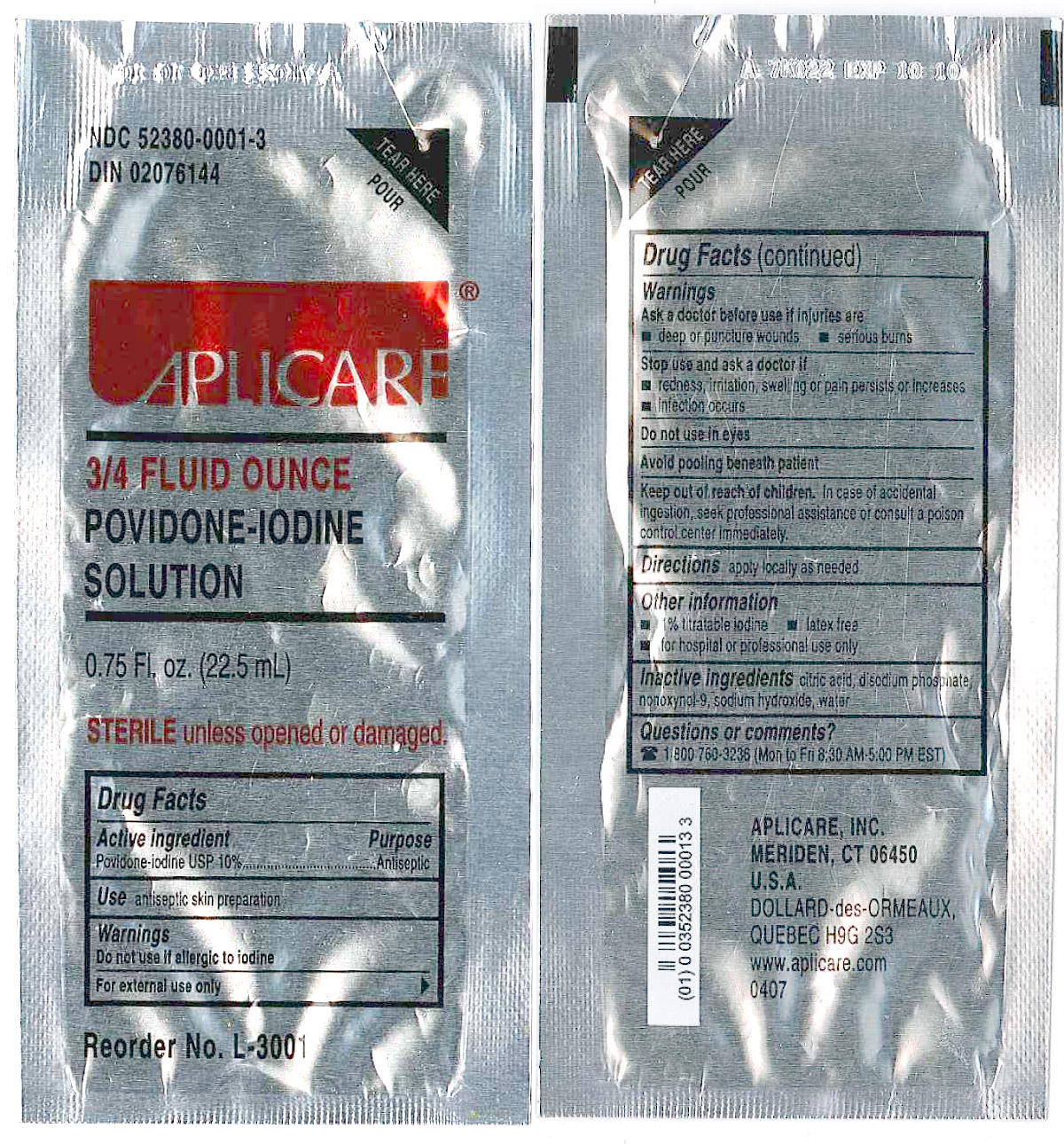
- DESCRIPTION
-
WARNINGS
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children. In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACL11KC NERVE BLOCK
regional anesthesia kit kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:55553-435 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:55553-435-02 24 in 1 CASE 1 1 in 1 PACKAGE, COMBINATION Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 22.5 mL Part 1 of 1 APLICARE POVIDONE-IODINE
povidone-iodine solutionProduct Information Item Code (Source) NDC: 52380-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52380-0001-3 22.5 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/01/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K965017 12/14/2005 Labeler - Clint Pharmaceuticals, Inc. (609197785) Registrant - Smiths Medical ASD, Inc. (137835299) Establishment Name Address ID/FEI Business Operations Smiths Medical ASD, Inc. 137835299 relabel, manufacture Establishment Name Address ID/FEI Business Operations Aplicare, Inc. 107255002 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.