viadur- leuprolide acetate
Drug Labeling and Warnings
Drug Details [pdf]
-
DESCRIPTION
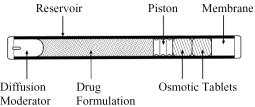
Viadur® (leuprolide acetate implant) is a sterile nonbiodegradable, osmotically driven miniaturized implant designed to deliver leuprolide acetate for 12 months at a controlled rate (Figure A). Viadur® incorporates DUROS® technology. The system contains 65 mg of leuprolide (free base). Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin-releasing hormone (GnRH or LH-RH). The analog possesses greater potency than the natural hormone. The implant is inserted subcutaneously in the inner aspect of the upper arm. After 12 months, the implant must be removed. At the time an implant is removed, another implant may be inserted to continue therapy.
Viadur® contains 72 mg of leuprolide acetate (equivalent to 65 mg leuprolide free base) dissolved in 104 mg dimethyl sulfoxide. The 4 mm by 45 mm titanium alloy reservoir houses a polyurethane rate-controlling membrane, an elastomeric piston, and a polyethylene diffusion moderator. The reservoir also contains the osmotic tablets, which are not released with the drug formulation. The osmotic tablets are composed of sodium chloride, sodium carboxymethyl cellulose, povidone, magnesium stearate, and sterile water for injection. Polyethylene glycol fills the space between the osmotic tablets and the reservoir. A minute amount of silicone medical fluid is used during manufacture as a lubricant. The weight of the implant is approximately 1.1g.
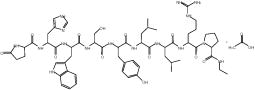
The chemical name is 5-Oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt), with the following structural formula:
-
CLINICAL PHARMACOLOGY
Leuprolide acetate, an LH-RH agonist, acts as a potent inhibitor of gonadotropin secretion when given continuously and in therapeutic doses. Animal and human studies indicate that after an initial stimulation, chronic administration of leuprolide acetate results in suppression of ovarian and testicular steroidogenesis.
In humans, administration of leuprolide acetate results in an initial increase in circulating levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to a transient increase in concentrations of gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in premenopausal females). However, continuous administration of leuprolide acetate results in decreased levels of LH and FSH. In males, testosterone is reduced to castrate levels. These decreases occur within 2 to 4 weeks after initiation of treatment.
One Viadur® Implant nominally delivers 120 micrograms of leuprolide acetate per day over 12 months. Leuprolide acetate is not active when given orally.
PHARMACOKINETICS
Absorption
After insertion of Viadur®, mean serum leuprolide concentrations were 16.9 ng/mL at 4 hours and 2.4 ng/mL at 24 hours. Thereafter, leuprolide was released at a constant rate. Mean serum leuprolide concentrations were maintained at 0.9 ng/mL (0.3 to 3.1 ng/mL; SD = ±0.4) for 12 months. Upon removal and insertion of a new Viadur® at 12 months, steady-state serum leuprolide concentrations were maintained.
Distribution
The mean steady-state volume of distribution of leuprolide following 1 mg intravenous (IV) bolus administration to healthy male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.1
Metabolism
In healthy male volunteers administered a 1 mg IV bolus of leuprolide, the mean systemic clearance was 8.34 L/h, with a terminal elimination half-life of approximately 3 hours, based on a two-compartment model.1
A pentapeptide (M-1) is the major leuprolide metabolite upon administration with different leuprolide acetate formulations. No drug metabolism study was conducted with Viadur®.
Dose Proportionality
In a study comparing one Viadur® implant to two Viadur® implants, mean serum leuprolide concentrations were proportional to dose.
Special Populations
Pediatrics
The safety and effectiveness of Viadur® in pediatric patients have not been established (see CONTRAINDICATIONS).
Race
In the patients studied (80 Caucasian, 23 Black, 3 Hispanic), mean serum leuprolide concentrations were similar.
-
CLINICAL STUDIES
In two open-label, non-comparative, multicenter studies, 131 patients with prostatic cancer were treated with Viadur® and evaluated for up to two years. Two-thirds of the patients had stage C or less advanced disease. The dose-ranging study assessed serum testosterone as the primary efficacy endpoint in 51 patients treated with either one [n=27] or two [n=24] implants for 12 months. The confirmatory study evaluated achievement and maintenance of serum testosterone suppression in 80 patients each treated with one implant for 12 months. Both studies included a removal procedure and insertion of a new implant with evaluation for 12 additional months.
Following the initial insertion in patients receiving one implant, mean serum testosterone concentrations increased from 422 ng/dL at baseline to 690 ng/dL on Day 3, then decreased to below baseline by week two (Figure B). Serum testosterone decreased below the 50 ng/dL castrate threshold by week four in all but one patient [106 of 107 patients, 99%]. Once serum testosterone suppression was achieved [one patient was not continuously suppressed until week 28], testosterone remained suppressed below the castrate threshold for the duration of the treatment phase.
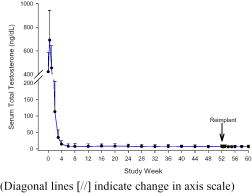
Figure B Mean (+SD) Serum Total Testosterone Concentrations – All Patients (n=107) Who Received One Implant
Most patients [n=118] had a new implant inserted for a second year of therapy following removal of the first implant(s). No patient experienced a clinically significant increase in serum testosterone [acute-on-chronic phenomenon] upon removal of the original implant(s) and insertion of a new implant. Suppression of serum testosterone was maintained in all patients through the two-month follow-up period following removal of the first implant(s) and insertion of a new implant.
Serum Prostate Specific Antigen (PSA) was monitored as a secondary endpoint in the clinical studies with Viadur®. Serum PSA decreased in all patients after they began treatment with Viadur®. At six months, PSA concentrations decreased from baseline by at least 90% in 74.2% of the 97 evaluable patients.
Periodic monitoring of serum testosterone and PSA concentrations is recommended, especially if the anticipated clinical or biochemical response to treatment has not been achieved.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
- Viadur® is contraindicated in patients with hypersensitivity to GnRH, GnRH agonist analogs, or any of the components in Viadur®. Anaphylactic reactions to synthetic GnRH or GnRH agonist analogs have been reported in the literature.2
- Viadur® is contraindicated in women and in pediatric patients and was not studied in women or children. Moreover, leuprolide acetate can cause fetal harm when administered to a pregnant woman. Major fetal abnormalities were observed in rabbits but not in rats after administration of leuprolide acetate throughout gestation. There were increased fetal mortality and decreased fetal weights in rats and rabbits. The effects on fetal mortality are expected consequences of the alterations in hormonal levels brought about by this drug. The possibility exists that spontaneous abortion may occur.
-
WARNINGS
Viadur®, like other LH-RH agonists, causes a transient increase in serum concentrations of testosterone during the first week of treatment. Patients may experience worsening of symptoms or onset of new symptoms, including bone pain, neuropathy, hematuria, or ureteral or bladder outlet obstruction (see PRECAUTIONS).
Cases of ureteral obstruction and spinal cord compression, which may contribute to paralysis with or without fatal complications, have been reported with LH-RH agonists.
If spinal cord compression or renal impairment develops, standard treatment of these complications should be instituted.
-
PRECAUTIONS
General
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy (see WARNINGS).
X-rays do not affect Viadur® functionality. Viadur® is radio-opaque and is well visualized on X-rays.
The titanium alloy reservoir of Viadur® is nonferromagnetic and is not affected by magnetic resonance imaging (MRI). Slight image distortion around Viadur® may occur during MRI procedures.
Laboratory tests
Response to Viadur® should be monitored by measuring serum concentrations of testosterone and prostate-specific antigen periodically.
Results of testosterone determinations are dependent on assay methodology. It is advisable to be aware of the type and precision of the assay methodology to make appropriate clinical and therapeutic decisions.
Drug/Laboratory Test Interactions
Therapy with leuprolide results in suppression of the pituitary-gonadal system. Results of diagnostic tests of pituitary gonadotropic and gonadal functions conducted during and after leuprolide therapy may be affected.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in rats and mice. In rats, dose-related increases of benign pituitary hyperplasia and benign pituitary adenomas were noted at 24 months when the drug was administered subcutaneously at high daily doses (4 to 24 mg/m2, 50 to 300 times the daily human exposure based on body surface area). There were significant but not dose-related increases of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice no pituitary abnormalities were observed at up to 180 mg/m2 (over 2000 times the daily human exposure based on body surface area) for 2 years.
Mutagenicity studies were performed with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of a mutagenic potential.
Pediatric Use
Viadur® is contraindicated in pediatric patients and was not studied in children
(see CONTRAINDICATIONS).
-
ADVERSE REACTIONS
The safety of Viadur® was evaluated in 131 patients with prostate cancer treated for up to 24 months in two clinical trials. Viadur®, like other LHRH analogs, caused a transient increase in serum testosterone concentrations during the first 2 weeks of treatment. Therefore, potential exacerbations of signs and symptoms of the disease during the first few weeks of treatment are of concern in patients with vertebral metastases and/or urinary obstruction or hematuria. If these conditions are aggravated, it may lead to neurological problems such as weakness and/or paresthesia of the lower limbs or worsening of urinary symptoms (see WARNINGS and PRECAUTIONS).
In the above-described clinical trials, the transient increase in serum testosterone concentrations was associated with an exacerbation of disease symptoms, manifested by pain or bladder outlet obstructive symptoms (urinary retention or frequency) in 6 (4.6%) patients.
The majority of local reactions associated with initial insertion or removal and insertion of a new implant began and resolved within the first two weeks. Reactions persisted in 9.3% of patients. 10.3% of patients developed application-site reactions after the first two weeks following insertion.
Local reactions after initial insertion of a single implant included bruising (34.6%) and burning (5.6%). Other, less frequently reported, reactions included pulling, pressure, itching, erythema, pain, edema, and bleeding.
In these two clinical trials, four patients had local infection/inflammations that resolved after treatment with oral antibiotics.
Local reactions following insertion of a subsequent implant were comparable to those seen after initial insertion.
In the first 12 months after initial insertion of the implant(s), an implant extruded through the incision site in three of 131 patients (see INSERTION AND REMOVAL PROCEDURES for correct implant placement).
The following possibly or probably related systemic adverse events occurred during clinical trials within 24 months of treatment with Viadur®, and were reported in ≥2% of patients (Table 1).
Table 1 Incidence (%) of Possibly or Probably Related Systemic Adverse Events Reported by ≥ 2% of Patients Treated with Viadur® for up to 24 Months * Expected pharmacologic consequences of testosterone suppression.
Body System
Adverse Event
Number (%)Body as a Whole Asthenia 10 (7.6%) Headache 6 (4.6%) Extremity pain 4 (3.1%)
Cardiovascular Vasodilatation (hot flashes)* 89 (67.9%)
Digestive Diarrhea 3 (2.3%)
Hematology and Lymphatic Ecchymosis 6 (4.6%) Anemia 3 (2.3%)
Metabolic and Nutritional Peripheral edema 4 (3.1%) Weight gain 3 (2.3%)
Nervous Depression 7 (5.3%)
Respiratory Dyspnea 3 (2.3%)
Skin Sweating* 7 (5.3%) Alopecia 3 (2.3%)
Urogenital Gynecomastia/breast enlargement* 9 (6.9%) Nocturia 5 (3.8%) Urinary frequency 5 (3.8%) Testis atrophy or pain* 5 (3.8%) Breast pain* 4 (3.1%) Impotence* 3 (2.3%) In addition, the following possibly or probably related systemic adverse events were reported by <2% of patients using Viadur® in clinical studies.
General: General pain, chills, abdominal pain, malaise, dry mucous membranes
Gastrointestinal: Constipation, nausea
Hematologic: Iron deficiency anemia
Metabolic: Edema, weight loss
Musculoskeletal: Bone pain, arthritis
Nervous: Dizziness, insomnia, paresthesia, amnesia, anxiety
Skin: Pruritus, rash, hirsutism
Urogenital: Urinary urgency, prostatic disorder, urinary tract infection, dysuria, urinary incontinence, urinary retention
Changes in Bone Density
Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with an LH-RH agonist analog. In a clinical trial, 25 men with prostate cancer, 12 of whom had been treated previously with leuprolide acetate for at least 6 months, underwent bone density studies as a result of pain. The leuprolide-treated group had lower bone density scores than the nontreated control group. It can be anticipated that long periods of medical castration in men will have effects on bone density.
Postmarketing
Pituitary apoplexy: During post-marketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
Ninety-seven of the 131 patients in the two-year duration studies that supported approval of Viadur® continued in an open-label, third-year extension study. One patient prematurely withdrew due to lack of efficacy that was attributed to a defective implant. Fifty of these patients continued in an open-label, fourth-year extension study. No spontaneous implant extrusions were reported in these extension studies. Since Viadur® has been commercially available, <1% of patients implanted have been reported to have a spontaneous implant extrusion (with or without associated infection).
Additional adverse events have been reported from US post-marketing experience with Viadur®. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been reported infrequently and include fatigue, hypertension, migration of implant, syncope, tremor, and vomiting.
-
OVERDOSAGE
In clinical trials using daily subcutaneous leuprolide acetate in patients with prostate cancer, doses as high as 20 mg/day for up to 2 years caused no adverse effects differing from those observed with the 1 mg/day dose. The adverse event profiles were similar in patients receiving one or two Viadur® implants.
-
DOSAGE AND ADMINISTRATION
The recommended dose of Viadur® is one implant for 12 months. Each implant contains 65 mg leuprolide. The implant is inserted subcutaneously in the inner aspect of the upper arm and provides continuous release of leuprolide for 12 months of hormonal therapy.
Viadur® must be removed after 12 months of therapy. At the time an implant is removed, another implant may be inserted to continue therapy. (See INSERTION AND REMOVAL PROCEDURES.)
Insertion and Removal Procedures
Viadur® is supplied in a box containing one sterile Viadur® implant in a sealed vial, one Viadur® sterile implanter, one sealed container of lidocaine HCl USP 2%, 10 mL, and one sterile Viadur® Kit. The Viadur® Kit is designed to provide a sterile field and supplies to facilitate the insertion and/or subsequent removal of the implant.
In addition to the Viadur® Kit, sterile gloves are required for the insertion procedure and subsequent removal of the implant.
Insertion Procedure
Under aseptic conditions, an implanter is used to place the implant under the skin.
The implant is inserted using the procedure outlined below.
Identifying the Insertion Site
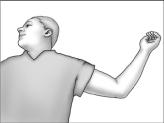
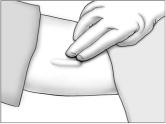
- Have the patient lie on his back on the examination table, with his left arm (if the patient is left-handed, the right arm) flexed at the elbow and externally rotated so that his hand is out to his side.
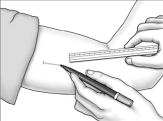
Using a pen and ruler, mark a site on the inner, upper arm approximately 8-10 cm above the elbow crease in the groove between the biceps and triceps muscles. Make sure that the site is unaffected by movement of the muscles.
Preparing the Sterile Field
- To establish a sterile field, carefully open the sterile Viadur® Kit. The sterile kit contains:
1 scalpel
1 forceps
1 syringe
1 package povidone-iodine swabs
1 package wound closure strips
1-22 Ga x 1.5” needle
1-25 Ga x 1.5” needle
6 gauze sponges
2 alcohol prep swabs
1 package skin protectant
1 bandage
1 fenestrated drape
1 marking pen
1 ruler
1 mosquito clamp
- The implant tray contains:
1 sealed vial, which contains the Viadur® implant
1 sterile implanter
1 sealed container of lidocaine HCl USP 2%, 10 mL
To open the vial, remove the metal band from the bottle and pull up the stopper. Carefully drop the implant from the bottle onto the sterile field. Then, carefully drop the implanter and the container of lidocaine onto the sterile field.
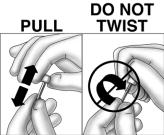
Using sterile technique, remove the protective cap from the implant by pulling the cap straight off. DO NOT TWIST CAP OFF AS IT MAY UNSCREW THE DIFFUSION MODERATOR, CAUSE ITS REMOVAL, OR OTHERWISE DAMAGE THE IMPLANT. SHOULD DAMAGE OCCUR, DO NOT INSERT THE IMPLANT AS PRODUCT FUNCTION CAN BE IMPAIRED.
Loading the Implanter
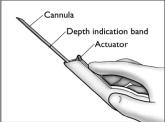
- The implanter is packaged in the correct configuration for implant loading and insertion. Make sure the cannula is fully extended as shown, and the actuator is in its most forward position.
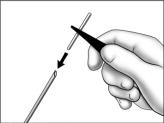
- Using sterile forceps, slide the implant into the end of the cannula and push until it stops. When properly loaded, the implant should not protrude more than 1 mm past the bottom of the beveled edge.
Inserting the Implant
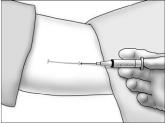
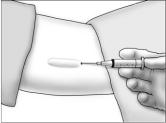
- Using aseptic technique, cleanse the insertion site, then drape the patient's arm. After determining the absence of known allergies to the anesthetic agent, infiltrate the site with lidocaine. Advance the needle to infiltrate the intended 5 cm track for the implant insertion.
- Determine that anesthesia is adequate. Make an incision of approximately 5 mm with the scalpel, just through the dermis.
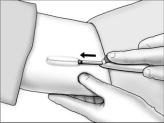
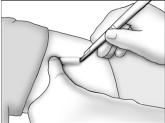
- Grasp the handle of the implanter and extend the index finger to rest on the back of the actuator as shown. Insert the cannula tip into the incision with the bevel up and advance it subcutaneously along the intended track. To ensure subcutaneous placement, the Viadur® implanter should visibly raise the skin at all times during insertion. The implanter should not enter muscle tissue, but be well within the subcutaneous space. Advance the implanter to the depth indicator on the cannula, which indicates the recommended insertion length.
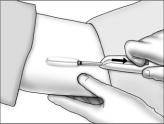
- Holding the implanter handle in position, use the index finger to slide the actuator slowly back until it stops. (This retracts the actuator cannula into the handle, leaving the implant beneath the skin.). Do not pull back on the implanter handle while sliding the actuator back, as this may lead to incorrect positioning of the implant and subsequent extrusion. Withdraw the implanter from the incision. Release of the implant can be checked by palpation. It is important to keep the implanter steady and not to push the implant into the tissue. After placement, sterile gauze may be used to apply pressure briefly to the insertion site to ensure hemostasis.
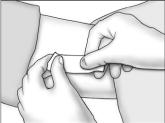
- Cleanse the insertion area. Press the edges of the incision together, and tightly close the incision with one or two surgical closure strips. Cover with an adhesive bandage. Observe the patient for a few minutes for signs of bleeding from the incision before he is discharged. Instruct the patient to keep the area clean and dry for 24 hours, and to avoid heavy lifting and strenuous physical activity for 48 hours. The surgical closure strip can be removed as soon as the incision has healed, ie, normally in 3 days.
Removal Procedure
Viadur® must be removed following 12 months of therapy.
The position of the patient and the sterile technique are the same as for insertion.
To remove Viadur® use the Viadur® Kit or the following sterile items:
- 1 scalpel
- 1 forceps
- 1 syringe
- 1 package povidone-iodine swabs
- 1 package wound closure strips
- 1-22 Ga x 1.5” needle
- 1-25 Ga x 1.5” needle
- 1 sealed container of lidocaine HCl USP 2%, 10 mL
- 6 gauze sponges
- 2 alcohol prep swabs
- 1 package skin protectant
- 1 bandage
- 1 fenestrated drape
- 1 marking pen
- 1 ruler
- 1 mosquito clamp
Preparing the Site
- Inspect the site, palpating the location of the implant. Mark the position of the implant with marking pen. Cleanse with povidone-iodine swab. Drape the area with a fenestrated drape.
Suggestion:If unable to locate by palpation, radiological imaging may be helpful.
- After determining the absence of known allergies to the anesthetic agent, apply a small amount of local anesthetic under the end of the implant nearest the original incision site. Then advance the needle to infiltrate the tissue along the track.
Removing the Implant
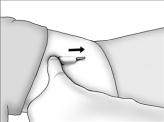
- Determine that anesthesia is adequate. Apply pressure to one end of the implant to elevate the other end. Make an incision of approximately 5 mm at the elevated end of the implant. Do not make a large incision.
Continue to apply pressure to the end of the implant to encourage expulsion. Push the implant gently towards the incision with the fingers. When the tip is visible or near the incision, grasp it with a clamp and remove.
- If necessary, cut through any fibrous encapsulation with the scalpel to free the implant.
- Properly dispose of removed implant immediately, before opening the vial containing the new implant.
If inserting a new Viadur®, return to section describing INSERTION PROCEDURE.
The new Viadur® implant may be placed through the same incision site. Alternatively, the contralateral arm may be used.
- Cleanse insertion site area. Apply pressure to each end of the incision to close the wound. Apply one or two surgical closure strips to close the wound tightly, and cover with an adhesive bandage. Observe the patient for a few minutes for signs of bleeding from the incision before he is discharged. Instruct the patient to keep the area clean and dry for 24 hours, and to avoid strenuous physical activity for 48 hours.
- Have the patient lie on his back on the examination table, with his left arm (if the patient is left-handed, the right arm) flexed at the elbow and externally rotated so that his hand is out to his side.
-
HOW SUPPLIED
Viadur® is supplied in a box containing 2 inner package trays. One tray contains a sterile Viadur® implant in a sealed vial, a sterile Viadur® implanter and a sealed container of lidocaine HCl USP 2%, 10 mL. The other tray constitutes a sterile Viadur® Kit, which includes: 1 scalpel, 1 forceps, 1 syringe, povidone-iodine swabs, 1 package wound closure strips, 1-22 Ga x 1.5” needle, 1-25 Ga x 1.5” needle, 6 gauze sponges, 2 alcohol prep swabs, 1 package skin protectant, 1 bandage, 1 fenestrated drape, 1 marking pen, 1 ruler, and 1 mosquito clamp. A physician insert, patient information, and insertion and removal instructions are also provided in the box.
(NDC: 0026-9711-01)
Rx Only
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). [see USP Controlled Room Temperature]
For more information call 1-800-288-8371 or visit www.VIADUR.com.
-
REFERENCES
- Sennello LT et al. Single-dose pharmacokinetics of leuprolide in humans following intravenous and subcutaneous administration. J Pharm Sci 1986; 75(2): 158-160.
- MacLeod TL et al. Anaphylactic reaction to synthetic luteinizing hormone-releasing hormone. Fertil Steril 1987; 48(3): 500-502.
Manufactured by:
ALZA Corporation
Mountain View, CA 94043 U.S.A.Distributed by:
Bayer Pharmaceuticals Corporation
400 Morgan Lane, West Haven, CT 06516 USAViadur® and DUROS® are registered trademarks of ALZA Corporation under license to Bayer Pharmaceuticals Corporation. For further information about the product contact Bayer Pharmaceuticals Corporation.
An ALZA DUROS® Technology Product
Edition Date: November 2005
-
SUPPLEMENTAL PATIENT MATERIAL
PATIENT INFORMATION ABOUT:
Viadur®(leuprolide acetate implant)
Important Information for Patients Using Viadur® for the treatment of symptoms of advanced prostate cancer.
Please read this information before you start using Viadur®. Each time another Viadur® is inserted, check the patient information leaflet for any new information. Remember, this information does not take the place of your doctor's instructions. Ask your doctor or pharmacist if you have questions or want more information about Viadur®.
What is Viadur®?
Viadur® is a drug-delivery system that contains the drug leuprolide and is placed under the skin. It looks like a small, thin metal tube. After it is placed under the skin, Viadur® delivers leuprolide to your body continuously for 12 months.
How does Viadur®work?
Leuprolide, the active medication in Viadur®, works by reducing the testosterone produced by the testicles. This lowers the amount of testosterone in the body. Testosterone appears to be needed by prostate cancer cells. Usually prostate cancer shrinks or stops growing when the body's supply of testosterone is lowered.
By lowering the amount of testosterone in the body, Viadur® may help relieve the pain, urinary problems, and other symptoms of prostate cancer. However, Viadur® is not a cure for prostate cancer. Once Viadur® is removed by your doctor, your body will start producing testosterone again.
How is Viadur®given?
Viadur® will be placed under the skin of your upper, inner arm. The doctor will numb your arm, make a small incision, and then place Viadur® under the skin. The incision will be closed with special surgical tape and covered with a bandage. You should keep the bandage in place for a few days until the incision heals.
After 12 months, Viadur® must be removed and may be replaced with a new Viadur® by your doctor.
What should I avoid while Viadur®is inserted?
After Viadur® is inserted, keep the site clean and dry for 24 hours. Do not bathe or swim for 24 hours. Avoid heavy lifting and physical activity for 48 hours. Avoid bumping the site for a few days. After the cut has healed, you should be able to go back to your normal activities.
What should I know about using Viadur®?
- If you notice unusual bleeding, redness or pain at the insertion site, contact your doctor.
- In the first few weeks of treatment, if you experience increased pain throughout your body, weakness, or numbness, contact your doctor.
- X-rays and MRI do not affect Viadur®.
- Viadur® is seen on X-rays. Slight image distortion around Viadur® may occur during MRI procedures.
- Viadur® must be removed and may be replaced after 12 months.
Who should NOT use Viadur®?
Do not use Viadur® if you are allergic to the drug leuprolide.
Do not use Viadur® if you are a woman. Viadur® is not approved for use by women of any age. Furthermore, use of Viadur® in a woman who is or may become pregnant may cause harm to the baby. You may lose your baby through a miscarriage if the drug is used while you are pregnant.
Viadur® was not studied in children and should not be used in children.
What are the most common side effects of Viadur®?
The most common side effects related to Viadur® were hot flashes, lack of energy, depression, sweating, headache, bruising, and breast enlargement.
Prostate cancer-related symptoms may become worse during the first few weeks of treatment.
Like other similar treatment options, Viadur® may cause impotence.
There may be some pain and discomfort during and after Viadur® insertion and removal. Bruising may occur. Reactions, such as itching and redness, are usually mild and heal without treatment within two weeks. If they do not heal, contact your doctor.
There is a chance that your bones may become thinner if you use this type of drug for long periods of time. Ask your doctor if this is a risk for you.
This list is not a complete list of all the possible side effects. If you need more information, or are worried about these or other side effects, talk to your doctor or pharmacists.
What tests will my doctor perform during my treatment with Viadur®?
Your doctor may measure blood levels of testosterone and prostate-specific antigen (PSA) during your treatment with Viadur®.
Can I take Viadur®with other medications?
Tell your doctor or pharmacist about any medicines that you are taking, including Viadur®, prescription, non-prescription, and herbal remedies. Do not start taking a new medicine before checking with your doctor or pharmacist.
For more information on Viadur®, talk to your doctor or pharmacist, call 1-800-288-8371 between 8:30 AM and 5 PM Eastern Standard Time, or visit www.VIADUR.com on the Internet.
-
INGREDIENTS AND APPEARANCE
VIADUR
leuprolide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0026-9711 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0026-9711-01 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 1 Part 2 1 10 mL Part 1 of 2 VIADUR
leuprolide acetate implantProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEUPROLIDE ACETATE (UNII: 37JNS02E7V) (LEUPROLIDE - UNII:EFY6W0M8TG) 72 mg Inactive Ingredients Ingredient Name Strength Dimethyl Sulfoxide (UNII: YOW8V9698H) magnesium stearate (UNII: 70097M6I30) Polyethylene glycol () Povidone () Silicone () Sodium Carboxymethyl Cellulose () sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 VIAL Part 2 of 2 LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride liquidProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine hydrochloride (UNII: V13007Z41A) (lidocaine - UNII:98PI200987) 20 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 CONTAINER Labeler - Bayer Pharmaceuticals Corporation
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.