NEOVA DNA DAMAGE CONTROL - ACTIVE BROAD SPECTRUM SPF 43- octinoxate, zinc oxide emulsion
Neova DNA Damage Control - Active by
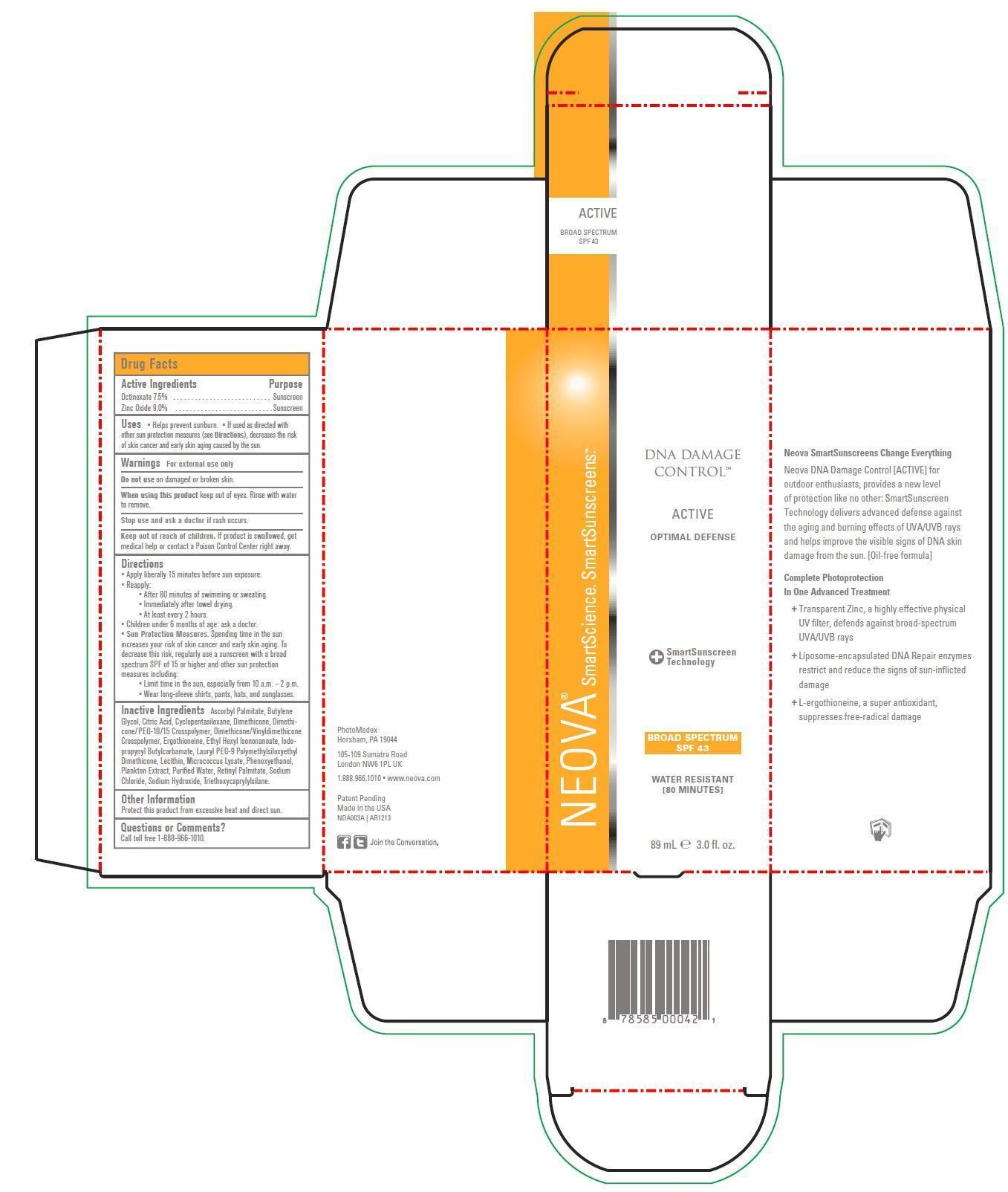
Drug Labeling and Warnings
Neova DNA Damage Control - Active by is a Otc medication manufactured, distributed, or labeled by PhotoMedex, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply:
°After 80 minutes of swimming or sweating.
°Immediately after towel drying.
°At least every two hours.
- Children under 6 months of age: ask a doctor.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
°Limit time in the sun, especially from 10 a.m. - 2 p.m.
°Wear long-sleeve shirts, pants, hats, and sunglasses.
-
Inactive Ingredients
Ascorbyl Palmitate, Butylene Glycol, Citric Acid, Cyclopentasiloxane, Dimethicone, Dimethicone/PEG-10/15 Crosspolymer, Dimethicone/Vinyldimethicone Crosspolymer, Ergothioneine, Ethyl Hexyl Isononanoate,Iodopropynyl Butylcarbamate, Lauryl PEG-9 Polymethylsiloxyethyl Dimethicone, Lecithin, Microcoous Lysate, Phenoxyethanol, Plankton Extract, Purified Water, Retinyl Palmitate, Sodium Chloride, Sodium Hydroxide, Triethanoxycaprylylsilane.
- Other Information
- Questions or Comments?
- Neova DNA Damage Control Active SPF 433.0 fl. oz. (89mL)
-
INGREDIENTS AND APPEARANCE
NEOVA DNA DAMAGE CONTROL - ACTIVE BROAD SPECTRUM SPF 43
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62362-179 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 9 g in 100 mL Inactive Ingredients Ingredient Name Strength ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) ERGOTHIONEINE (UNII: BDZ3DQM98W) ETHYLHEXYL ISONONANOATE (UNII: I6KB4GE3K4) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62362-179-01 1 in 1 BOX 1 NDC: 62362-179-89 89 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/08/2012 Labeler - PhotoMedex, Inc. (054503875) Establishment Name Address ID/FEI Business Operations PhotoMedex, Inc. 054503875 manufacture(62362-179)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 DNADamageControl3ozActiveLabel.jpg
DNADamageControl3ozActiveLabel.jpg