LOQTORZI- toripalimab-tpzi injection
LOQTORZI by
Drug Labeling and Warnings
LOQTORZI by is a Prescription medication manufactured, distributed, or labeled by Coherus BioSciences, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LOQTORZI safely and effectively. See full prescribing information for LOQTORZI.
LOQTORZI® (toripalimab-tpzi) injection, for intravenous use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
LOQTORZI is a programmed death receptor-1 (PD-1)- blocking antibody indicated:
- in combination with cisplatin and gemcitabine, for first-line treatment of adults with metastatic or with recurrent locally advanced nasopharyngeal carcinoma (NPC) (1.1)
- as a single agent for the treatment of adults with recurrent unresectable or metastatic NPC with disease progression on or after a platinum-containing chemotherapy (1.2)
DOSAGE AND ADMINISTRATION
In combination with cisplatin and gemcitabine:
- 240 mg intravenously every three weeks (2.1)
As a single agent:
- 3 mg/kg intravenously every two weeks (2.1)
First Infusion: Infuse over 60 minutes.
Subsequent Infusions: If no infusion-related reactions occurred during the first infusion, subsequent infusions may be administered over 30 minutes.
DOSAGE FORMS AND STRENGTHS
Injection: 240 mg/6 mL (40 mg/mL) solution in a single-dose vial (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
-
Immune-Mediated Adverse Reactions (5.1)
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, immune-mediated dermatologic adverse reactions, and solid organ transplant rejection.
- Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. (5.1)
- Withhold or permanently discontinue based on severity and type of reaction. (5.1)
- Infusion-related reactions: Interrupt, slow the rate of infusion, or permanently discontinue LOQTORZI based on the severity of reaction. (5.2)
- Complications of allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. (5.3)
- Embryo-fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective method of contraception. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
-
LOQTORZI in Combination with Cisplatin and Gemcitabine
The most common adverse reactions (≥ 20%) are nausea, vomiting, decreased appetite, constipation, hypothyroidism, rash, pyrexia, diarrhea, peripheral neuropathy, cough, musculoskeletal pain, upper respiratory infection, insomnia, dizziness, and malaise. (6.1) -
LOQTORZI as a Single Agent
The most common adverse reactions (≥ 20%) are fatigue, hypothyroidism and musculoskeletal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Coherus Oncology, Inc. at 1-800-483-3692 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 First-line Treatment of Metastatic or Recurrent, Locally Advanced NPC with Cisplatin and Gemcitabine
1.2 Previously Treated Unresectable or Metastatic NPC
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
5.2 Infusion-Related Reactions
5.3 Complications of Allogeneic HSCT
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 First-line Treatment of Metastatic or Recurrent, Locally Advanced NPC with Cisplatin and Gemcitabine
14.2 Previously Treated Unresectable or Metastatic NPC
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosages of LOQTORZI are provided in Table 1. Administer as recommended [see Dosage and Administration (2.3)].
Table 1: Recommended Dosage Indication Recommended Dosage of LOQTORZI Duration of Treatment First-line NPC 240 mg every three weeks Until disease progression, unacceptable toxicity, or up to 24 months. Recurrent NPC 3 mg/kg every two weeks Until disease progression or unacceptable toxicity. 2.2 Dosage Modifications
No dose reductions of LOQTORZI are recommended. In general, withhold LOQTORZI for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue LOQTORZI for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce prednisone to 10 mg per day or less (or equivalent) within 12 weeks of initiating steroids.
Dosage modifications for LOQTORZI for adverse reactions that require management different from these general guidelines are summarized in Table 2.
Table 2: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction Severity* Dose Modification ALT=alanine aminotransferase, AST=aspartate aminotransferase, DRESS=drug rash with eosinophilia and systemic symptoms, SJS=Stevens Johnson syndrome, TEN=toxic epidermal necrolysis, ULN=upper limit of normal - * Based on National Cancer Institute (NCI) Common Terminology for Adverse Events (CTCAE) version 5.0
- † Resume LOQTORZI in patients with complete or partial resolution to Grade 0-1 after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating steroids or inability to reduce prednisone to 10 mg per day or less (or equivalent) within 12 weeks of initiating steroids.
- ‡ If AST and ALT are less than or equal to ULN at baseline in patients with liver involvement, withhold or permanently discontinue LOQTORZI based on recommendations for hepatitis with no liver involvement.
Immune-Related Adverse Reactions [see Warnings and Precautions (5.1)] Pneumonitis Grade 2 Withhold† Grades 3 or 4 Permanently discontinue Colitis Grade 2 or 3 Withhold† Grade 4 Permanently discontinue Hepatitis with no tumor involvement of the liver AST/ALT increases to more than 3 and up to 8 times ULN
or
Total bilirubin increases to more than 1.5 and up to 3 times ULNWithhold† AST or ALT increases to more than 8 times ULN
or
Total bilirubin increases to more than 3 times ULNPermanently discontinue Hepatitis with tumor involvement of the liver‡ Baseline AST or ALT is more than 1 and up to 3 times ULN and increases to more than 5 and up to 10 times ULN
or
Baseline AST or ALT is more than 3 and up to 5 times ULN and increases to more than 8 and up to 10 times ULNWithhold† Baseline AST or ALT is above the ULN and increases to more than 10 times ULN
or
Total bilirubin increases to more than 3 times ULNPermanently discontinue Endocrinopathies Grades 3 or 4 Withhold until clinically stable or permanently discontinue depending on severity† Nephritis with Renal Dysfunction Grade 2 or 3 increased blood creatinine Withhold† Grade 4 increased blood creatinine Permanently discontinue Exfoliative Dermatologic Conditions Suspected SJS, TEN, or DRESS Withhold† Confirmed SJS, TEN, or DRESS Permanently discontinue Myocarditis Grades 2, 3, or 4 Permanently discontinue Neurological toxicities Grade 2 Withhold† Grade 3-4 Permanently discontinue Other Adverse Reactions Infusion-related reactions
[see Warnings and Precautions (5.2)]Grade 1 or 2 Interrupt or slow the rate of infusion Grade 3 or 4 Stop infusion
Permanently discontinue2.3 Preparation and Administration
Preparation for Intravenous Infusion
- Visually inspect the solution for particulate matter and discoloration. The solution is clear to slightly opalescent, colorless to slightly yellow. Discard the vial if visible particles are observed.
- Withdraw the required volume of LOQTORZI and inject slowly into a 100 mL or 250 mL infusion bag containing 0.9% Sodium Chloride Injection, USP. Mix diluted solution by gentle inversion. Do not shake. The final concentration of the diluted solution should be between 1 mg/mL to 3 mg/mL.
- Discard any unused portion left in the vial.
Storage of Diluted Solution for Infusion
LOQTORZI does not contain a preservative.
If the diluted solution is not administered immediately, store either:
- At room temperature, 20°C to 25°C (68°F to 77°F), for no more than 8 hours from the time of dilution to the completion of the infusion. Discard diluted solution stored at room temperature after 8 hours.
Or - Refrigerated at 2°C to 8°C (36°F to 46°F) for no more than 24 hours from the time of dilution to the completion of the infusion. If refrigerated, allow the diluted solution to come to room temperature prior to administration. Discard the refrigerated diluted solution after 24 hours.
Do not freeze.
Administration
- Administer diluted solution intravenously via an infusion pump using an in-line aseptic filter (0.2 or 0.22 micron).
- First Infusion: Infuse over at least 60 minutes.
- Subsequent infusions: If no infusion-related reactions occurred during the first infusion, subsequent infusions may be administered over 30 minutes [see Dose Modifications (2.2)].
- Do not co-administer other drugs through the same intravenous line.
- When administered on the same day as chemotherapy, LOQTORZI should be administered prior to chemotherapy.
- Refer to the Prescribing Information for cisplatin and gemcitabine for recommended dosing information.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
LOQTORZI is a monoclonal antibody that belongs to a class of drugs that bind to either the programmed death receptor-1 (PD-1) or PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed under WARNINGS AND PRECAUTIONS may not include all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time after starting PD-1/PD-L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue LOQTORZI depending on severity [see Dosage and Administration (2.2)]. In general, if LOQTORZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
LOQTORZI in Combination with Cisplatin and Gemcitabine
LOQTORZI in combination with cisplatin and gemcitabine can cause immune-mediated pneumonitis. In patients treated with other PD-1/PD-L1 blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation. Immune-mediated pneumonitis occurred in 2.1% (3/146) of patients receiving LOQTORZI, including Grade 2 (1.4%) adverse reactions. Pneumonitis resolved in 67% (2/3) of these patients.
LOQTORZI as a Single-Agent
LOQTORZI can cause immune-mediated pneumonitis. In patients treated with other PD-1/PD-L1 blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation. Immune-mediated pneumonitis occurred in 2.6% (22/851) of patients receiving LOQTORZI, including fatal (0.2%), Grade 3 (0.7%), and Grade 2 (1.1%) adverse reactions. Systemic corticosteroids were required in 82% (18/22) of patients with pneumonitis. Pneumonitis led to permanent discontinuation of LOQTORZI in 1.2% (10/851) of patients. Pneumonitis resolved in 23% (5/22) of these patients.
Immune-Mediated Colitis
LOQTORZI can cause immune-mediated colitis, which may present with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Hepatotoxicity and Immune-Mediated Hepatitis
LOQTORZI in Combination with Cisplatin and Gemcitabine
LOQTORZI in combination with cisplatin and gemcitabine can cause immune-mediated hepatitis. Immune-mediated hepatitis occurred in 0.7% (1/146) of patients receiving LOQTORZI in combination with cisplatin and gemcitabine, which was a Grade 3 (0.7%) adverse reaction. The patient with immune-mediated hepatitis required systemic corticosteroids.
LOQTORZI as a Single-Agent
LOQTORZI can cause immune-mediated hepatitis. Immune-mediated hepatitis occurred in 3.3% (28/851) of patients receiving LOQTORZI, including Grade 4 (0.8%), Grade 3 (2.1%), and Grade 2 (0.4%) adverse reactions. Hepatitis led to permanent discontinuation of LOQTORZI in 1.1% of patients and withholding of LOQTORZI in 0.8% of patients. Hepatitis resolved in 54% (15/28) of these patients.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency
LOQTORZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold or permanently discontinue LOQTORZI depending on severity [see Dosage and Administration (2.2)].
LOQTORZI as a Single-Agent
Adrenal insufficiency occurred in 0.5% (4/851) of the patients receiving LOQTORZI, including Grade 2 (0.4%) and Grade 1 (0.1%) adverse reactions. Systemic corticosteroids were required in 75% (3/4) of the patients with adrenal insufficiency. Adrenal insufficiency led to withholding of LOQTORZI in 0.1% (1/851) of patients. In the one patient in whom LOQTORZI was withheld, LOQTORZI was reinitiated after symptom improvement.
Hypophysitis
LOQTORZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effects such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism. Initiate hormone replacement as indicated. Withhold or permanently discontinue LOQTORZI depending on severity [see Dosage and Administration (2.2)].
LOQTORZI as a Single-Agent
Hypophysitis occurred in 0.4% (3/851) of patients receiving LOQTORZI, including Grade 3 (0.2%) and Grade 2 (0.1%) adverse reactions. All three patients received systemic corticosteroids. Hypophysitis led to permanent discontinuation of LOQTORZI in 0.1% (1/851) of patients and withholding of LOQTORZI in 0.1% (1/851) of patients. The one patient in whom LOQTORZI was withheld reinitiated LOQTORZI.
Thyroid Disorders
LOQTORZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated. Withhold or permanently discontinue LOQTORZI depending on severity [see Dosage and Administration (2.2)].
LOQTORZI in Combination with Cisplatin and Gemcitabine
Thyroiditis occurred in 2.1% (3/146) of patients receiving LOQTORZI in combination with cisplatin and gemcitabine, including Grade 2 (1.4%). Three patients required thyroid hormone replacement therapy. Thyroiditis resolved in one of the 3 patients.
Hyperthyroidism occurred in 1.4% (2/146) of patients receiving LOQTORZI in combination with cisplatin and gemcitabine. Hyperthyroidism resolved in these 2 patients.
Hypothyroidism occurred in 30% (44/146) of patients receiving LOQTORZI in combination with cisplatin and gemcitabine, including Grade 2 (24%) and Grade 1 (6%). Eighty percent of the 44 patients required thyroid hormone replacement therapy. LOQTORZI was withheld in 2.1% (3/146) of the patients. Of the 3 patients in whom LOQTORZI was withheld, 2 patients reinitiated LOQTORZI.
LOQTORZI as a Single-Agent
Thyroiditis occurred in 0.6% (5/851) of patients receiving LOQTORZI, including Grade 2 (0.1%). Two of these 5 patients received systemic corticosteroids and 2 required thyroid hormone replacement therapy. Thyroiditis resolved in 2 of the 5 patients.
Hyperthyroidism occurred in 7% (55/851) of patients receiving LOQTORZI, including Grade 2 (1.9%). Hyperthyroidism resolved in 85% (47/55) of the patients.
Hypothyroidism occurred in 15% (128/851) of patients receiving LOQTORZI, including Grade 2 (8%). Sixty three percent of the 128 patients required thyroid hormone replacement therapy. LOQTORZI was withheld in 0.5% of patients. Of the 4 patients in whom LOQTORZI was withheld, 3 patients reinitiated LOQTORZI.
Type 1 Diabetes Mellitus, which can present with Diabetic Ketoacidosis
Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold or permanently discontinue LOQTORZI depending on severity [see Dosage and Administration (2.2)].
LOQTORZI as a Single-Agent
Diabetes mellitus occurred in 0.9% (8/851) of patients receiving LOQTORZI, including Grade 4 (0.1%), Grade 3 (0.7%), and Grade 2 (0.1%). Diabetes mellitus led to permanent discontinuation in 0.4% of patients. Six of the 8 (75%) patients with diabetes mellitus required long-term insulin therapy.
Immune-Mediated Nephritis with Renal Dysfunction
LOQTORZI in Combination with Cisplatin and Gemcitabine
LOQTORZI in combination with cisplatin and gemcitabine can cause immune-mediated nephritis. Immune-mediated nephritis occurred in 0.7% (1/146) of patients receiving LOQTORZI. The one patient with immune-mediated nephritis (Grade 4) required systemic corticosteroids and nephritis led to discontinuation of LOQTORZI. Nephritis resolved in this patient.
Immune-Mediated Dermatologic Adverse Reactions
LOQTORZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue LOQTORZI depending on severity [see Dosage and Administration (2.2)].
LOQTORZI in Combination with Cisplatin and Gemcitabine
Immune-mediated dermatologic adverse reactions occurred in 8% (12/146) of patients receiving LOQTORZI, including Grade 3 (3.4%) and Grade 2 (1.4%) adverse reactions. Systemic corticosteroids were required in 25% (3/12) of the patients with immune-mediated dermatologic adverse reactions. Immune-mediated dermatologic adverse reactions led to permanent discontinuation of LOQTORZI in 2.1% (3) of patients. Immune-mediated dermatologic adverse reactions resolved in 92% (11/12) of these patients.
LOQTORZI as a Single-Agent
Immune-mediated dermatologic adverse reactions occurred in 4% (34/851) of patients receiving LOQTORZI, including Grade 3 (0.4%) and Grade 2 (1.4%) adverse reactions. Immune-mediated dermatologic adverse reactions led to withholding of LOQTORZI in 0.4% (3) of the patients. Systemic corticosteroids were required in 12% (4/34) of the patients with immune-mediated dermatologic adverse reactions. Immune-mediated dermatologic adverse reactions resolved in 71% (24/34) of these patients.
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received LOQTORZI or were reported with the use of other PD1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions.
Cardiac/Vascular: Myocarditis, pericarditis, vasculitis, pericardial effusion
Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy
Ocular: Uveitis, iritis and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis, to include increases in serum amylase and lipase levels, gastritis, duodenitis
Musculoskeletal and Connective Tissue: Myositis/polymyositis, rhabdomyolysis (and associated sequelae, including renal failure), arthritis, polymyalgia rheumatica, dermatomyositis
Endocrine: Hypoparathyroidism
Hematologic/Immune: Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection, other transplant (including corneal graft) rejection.
5.2 Infusion-Related Reactions
LOQTORZI can cause severe or life-threatening infusion-related reactions including hypersensitivity and anaphylaxis.
LOQTORZI in Combination with Cisplatin and Gemcitabine
Infusion-related reactions have been reported in 4.1% of patients receiving LOQTORZI in combination with cisplatin and gemcitabine, including Grade 2 (0.7%) reactions.
LOQTORZI as a Single-Agent
Infusion-related reactions occurred in 2% of 851 patients receiving LOQTORZI as single agent, including Grade 3 (0.1%) and Grade 2 (0.6%). LOQTORZI was withheld for one Grade 3 infusion related reaction.
Monitor patients for signs and symptoms of infusion-related reactions including rigors, chills, wheezing, pruritus, flushing, rash, hypotension, hypoxemia, and fever. Interrupt or slow the rate of infusion for mild (Grade 1) or moderate (Grade 2) infusion-related reactions. For severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions, stop infusion and permanently discontinue LOQTORZI [see Dosage and Administration (2.2)].
5.3 Complications of Allogeneic HSCT
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT.
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, LOQTORZI can cause fetal harm when administered to a pregnant woman. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death. Advise women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with LOQTORZI and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Severe and fatal immune-mediated adverse reactions [see Warnings and Precautions (5.1)]
- Infusion-related reactions [see Warnings and Precautions (5.2)]
- Complications of Allogeneic HSCT [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS section reflect exposure to LOQTORZI at a dose of 240 mg every 3 weeks in combination with up to 6 cycles of cisplatin and gemcitabine, followed by LOQTORZI 240 mg IV every 3 weeks, in 146 patients with NPC enrolled in a randomized, double-blind, placebo-controlled trial (JUPITER-02). Among the 146 patients, 73% were exposed to LOQTORZI for 6 months or more and 54% were exposed for 12 months or more. The most common adverse reactions (≥ 20%) were: nausea (71%), vomiting (68%), decreased appetite (55%), constipation (39%), hypothyroidism (38%), rash (36%), pyrexia (32%), diarrhea (31%), peripheral neuropathy (30%), cough (26%), musculoskeletal pain (25%), upper respiratory infection (23%), insomnia (23%), dizziness (21%), and malaise (21%). The most common Grade 3 or 4 laboratory abnormalities (≥2%) were: decreased neutrophils (58%), decreased lymphocytes (57%), decreased hemoglobin (50%) decreased platelets (33%), decreased potassium (10%), decreased sodium (9%), increased alanine aminotransferase (6%) increased or decreased magnesium (4.2% each), decreased calcium (3.5%), increased aspartate aminotransferase (2.7%), and bilirubin increased (2.1%).
The data described in the WARNINGS AND PRECAUTIONS section also reflects exposure to LOQTORZI as a single agent at a dose of 3 mg/kg IV every 2 weeks in 851 patients enrolled in 12 trials: one randomized, active-controlled trial and 11 open-label, non-randomized trials. The tumor types included nasopharyngeal carcinoma (n=193) or other types of tumors (n=658). Among the 851 patients treated with LOQTORZI as a single agent, 35% were exposed for 6 months or more and 20% were exposed for 12 months or more. In this pooled safety population, the most common (≥20%) adverse reactions were: fatigue (22%), hypothyroidism (20%), and musculoskeletal pain (20%). The most common Grade 3 or 4 laboratory abnormalities (≥2%), were decreased sodium (9%), decreased lymphocytes (8%), decreased hemoglobin (7%), decreased fibrinogen (4.5%), increased lipase (4.0%), increased amylase (2.9%), decreased phosphate (2.8%), increased aspartate aminotransferase (2.6%), increased glucose (2.5%), and increased triglycerides (2.1%).
First-line Treatment of Metastatic or Recurrent, Locally Advanced Nasopharyngeal Carcinoma (NPC)
The safety of LOQTORZI in combination with cisplatin and gemcitabine was evaluated in JUPITER-02 [see Clinical Studies (14)]. Key eligibility criteria were recurrent locally advanced or metastatic nasopharyngeal carcinoma (NPC) not previously treated with systemic chemotherapy for recurrent or metastatic disease. Patients with recurrent NPC after treatment with curative intent were required to have an interval of at least 6 months between the last dose of radiotherapy or chemotherapy and recurrence. Patients received LOQTORZI 240 mg (n=146) or placebo intravenously (IV) every 3 weeks (n=143), in combination with cisplatin 80 mg/m2 IV every 3 weeks and gemcitabine 1000 mg/m2 IV days 1 and 8 for up to 6 cycles followed by LOQTORZI 240 mg or placebo IV every 3 weeks until disease progression, unacceptable toxicity, or completion of 2 years of treatment. Among patients who received LOQTORZI, 73% were exposed for 6 months or longer and 54% were exposed for greater than one year.
The median age of patients who received LOQTORZI was 48 years (range: 19 to 72), 83% male, 100% Asian, 60% had recurrent disease, and 40% presented with metastatic disease. Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) was 0 (57%) or 1 (43%). Approximately 59% of patients had received at least one prior systemic therapy for locally advanced disease and 60% had received prior radiation therapy.
Serious adverse reactions occurred in 43% of patients receiving LOQTORZI in combination with cisplatin and gemcitabine. Serious adverse drug reactions in ≥ 2% were thrombocytopenia (14%), neutrophil count decreased (10%), pneumonia (10%), anemia (9%), abnormal hepatic function (2.7%), and rash (2.1%). Of the patients who received LOQTORZI in combination with cisplatin and gemcitabine, there were three fatal adverse reactions (2.1%) one due to epistaxis; one due to intracranial hemorrhage associated with immune-related thrombocytopenia and coagulopathy; and one due to pneumonia.
Permanent discontinuation of LOQTORZI, when given in combination with cisplatin and gemcitabine, due to an adverse reaction occurred in 12% of patients. Adverse reactions resulting in permanent discontinuation of LOQTORZI in ≥1% were pneumonia (2.1%), pulmonary tuberculosis (1.4%), rash (1.4%), and vomiting (1.4%).
Dosage interruptions of LOQTORZI due to an adverse reaction occurred in 50% of patients. Adverse reactions which required dosage interruption in ≥2% were anemia (17%), decreased neutrophils (12%), thrombocytopenia (12%), acute kidney injury (4.1%), pneumonia (6%), fatigue (2.7%), upper respiratory infection (2.7%), and hypothyroidism (2.1%).
Table 3 summarizes the adverse reactions in JUPITER-02.
Table 3: Adverse Reactions (≥ 10%) in Patients with Recurrent, Locally Advanced or Metastatic NPC Who Received LOQTORZI in Combination with Cisplatin and Gemcitabine in JUPITER-02 Adverse Reaction* LOQTORZI
Cisplatin/Gemcitabine
N = 146Placebo
Cisplatin/Gemcitabine
N = 143All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * NCI CTCAE v5.0.
- † Includes mouth ulceration, stomatitis, and radiation stomatitis.
- ‡ Includes hypothyroidism, tri-iodothyronine decreased, tri-iodothyronine free decreased, and thyroiditis.
- § Includes acneiform dermatitis, allergic dermatitis, catheter-site rash, dermatitis, drug eruption, eczema, erythema, macule, maculopapular rash, palmar-plantar erythrodysesthesia syndrome, papule, pruritic rash, rash, and urticaria.
- ¶ Includes asthenia and fatigue.
- # Includes hypoesthesia, neuralgia, neuropathy peripheral, paresthesia, peripheral sensory neuropathy.
- Þ Includes cough and productive cough.
- ß Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, pain in extremity, pain in jaw.
- à Includes acute sinusitis, bronchitis, laryngitis, nasopharyngitis, pharyngitis, respiratory tract infection, rhinitis, sinusitis, and upper respiratory tract infection.
- è Includes aspiration pneumonia and pneumonia
- ð Includes blood pressure increased, blood pressure systolic increased, hypertension, and hypertensive crisis.
Gastrointestinal Disorders Nausea 71 1.4 84 2.8 Vomiting 68 2.1 66 2.1 Constipation 39 0 46 0 Diarrhea 31 1.4 23 0 Stomatitis† 12 0 8 0.7 Metabolism and Nutrition Disorders Decreased appetite 55 0.7 63 0 Endocrine Disorders Hypothyroidism‡ 38 0.7 17 0 Skin Disorders Rash§ 36 3.4 28 2.8 Pruritus 17 0 8 0 General Disorders Pyrexia 32 1.4 24 0.7 Malaise 21 0.7 20 0 Fatigue¶ 19 0.7 22 2.1 Nervous System Disorders Peripheral neuropathy# 30 0 31 0.7 Dizziness 21 0 22 0.7 Headache 18 0 23 0.7 Respiratory, Thoracic and Mediastinal Disorders CoughÞ 26 0 27 0 Musculoskeletal Disorders Musculoskeletal painß 25 0 25 0.7 Infections Upper respiratory infectionà 23 3.4 13 2.8 Pneumoniaè 18 11 7 3.5 Psychiatric Disorders Insomnia 23 0 17 0 Vascular Disorders Epistaxis 10 1.3 13 2.8 Hypertensionð 10 6 6 4.2 Table 4 summarizes the laboratory abnormalities in JUPITER-02.
Table 4: Select Laboratory Abnormalities (≥20%) That Worsened from Baseline in Patients with Recurrent, Locally Advanced or Metastatic NPC Who Received LOQTORZI in Combination with Cisplatin and Gemcitabine in JUPITER-02 Laboratory Abnormalities* LOQTORZI
Cisplatin/GemcitabinePlacebo
Cisplatin/GemcitabineAll Grades†
(%)Grade 3 or 4
(%)All Grades
(%)Grades 3 or 4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: LOQTORZI/chemotherapy (range: 139 to 146 patients) and placebo/chemotherapy (range: 136 to 143 patients).
- † Graded per NCI CTCAE v5.0; AKP=alkaline phosphatase. ALT=alanine aminotransferase. AST=aspartate aminotransferase.
Hematology Decreased hemoglobin 94 50 97 38 Decreased neutrophils 91 58 95 63 Decreased lymphocytes 88 57 88 49 Decreased platelets 71 33 66 31 Chemistry Decreased magnesium 78 4.2 77 8 Decreased sodium 63 9 62 6 Increased alanine aminotransferase 58 6 50 3.5 Increased aspartate aminotransferase 58 2.7 53 4.9 Decreased albumin 49 0 48 0 Decreased calcium 45 3.5 46 4.2 Increased lactate dehydrogenase 42 0 35 0 Increased calcium 39 0 35 0.7 Decreased potassium 40 10 39 8 Increased creatinine 39 0.7 41 0 Increased alkaline phosphatase 27 0 27 0 Decreased glucose 23 1.4 16 0 Previously Treated, Unresectable or Metastatic Nasopharyngeal Carcinoma (NPC)
The safety of LOQTORZI was evaluated in POLARIS-02. Eligible patients had previously treated unresectable or metastatic NPC. Patients received LOQTORZI 3 mg/kg every 2 weeks as an intravenous infusion until disease progression or unacceptable toxicity. Among patients who received LOQTORZI, 33% were exposed for 6 months or longer and 21% were exposed for greater than one year.
The median age of patients who received LOQTORZI was 46 years (range: 22 to 71), 83% male, 100% Asian, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0 (35%) or 1 (65%) and median weight 59 kg (range: 32 to 101 kg).
Serious adverse reactions occurred in 24% of patients who received LOQTORZI. Serious adverse drug reactions in (≥2%) were pneumonia (4.7%), abnormal hepatic function (2.6%), and hyperbilirubinemia (2.1%). Fatal adverse reactions occurred in 3.7% of patients who received LOQTORZI, including death not otherwise specified (1.6%), tumor hemorrhage (0.5%), hepatic failure and thrombocytopenia (0.5%), hyponatremia (0.5%), and sudden death (0.5%).
Permanent discontinuation of LOQTORZI due to an adverse reaction occurred in 9% of patients. Adverse reaction resulting in permanent discontinuation of LOQTORZI in ≥1% included pneumonia (1.1%), abnormal hepatic function (1.1%), and hyperbilirubinemia (1.1%).
Dosage interruptions due to an adverse reaction occurred in 23% of patients. Adverse reactions which required dosage interruption in ≥1% were pneumonia (2.1%), thrombocytopenia (2.1%), fatigue (1.6%), hyperbilirubinemia (1.6%), anemia (1.1%), decreased appetite (1.1%), abnormal hepatic function (1.1%), hypothyroidism (1.1%), and pneumonitis (1.1%).
Table 5 summarizes the adverse reactions in POLARIS-02.
Table 5: Adverse Reactions (≥10%) in Patients with Previously Treated, Unresectable or Metastatic NPC Who Received LOQTORZI in POLARIS-02 Adverse Reaction* LOQTORZI
N=190All Grades
(%)Grade 3 or 4
(%)- * Toxicity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v4.03.
- † Includes hypothyroidism, thyroiditis, tri-iodothyronine decreased, and tri-iodothyronine free decreased
- ‡ Includes fatigue and asthenia
- § Includes cough and productive cough.
- ¶ Includes musculoskeletal pain and myalgia.
- # Includes dermatitis allergic, eczema, and rash.
Endocrine Disorders Hypothyroidism† 27 0 General Disorders Fatigue‡ 22 2.6 Pyrexia 16 0 Respiratory Disorders Cough§ 20 0 Musculoskeletal Disorders Musculoskeletal Pain¶ 18 1.1 Metabolism and Nutrition Decreased Appetite 13 1.1 Gastrointestinal Disorders Constipation 11 0 Skin and Subcutaneous Disorders Pruritus 11 0 Rash# 11 0 Investigations Weight Decreased 11 0 Table 6 summarizes the laboratory abnormalities in POLARIS-02.
Table 6: Select Laboratory Abnormalities (≥20%) That Worsened from Baseline in Patients with Previously Treated, Unresectable or Metastatic NPC Who Received LOQTORZI in POLARIS-02 LOQTORZI All Grades
(%)*Grade 3 or 4
(%)*- * Toxicity graded per NCI CTCAE v4.03. The denominator used to calculate the rate varied from 141 to 186 based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Decreased albumin 38 0.5 Decreased sodium 35 11 Decreased phosphate 32 3.2 Increased aspartate aminotransferase 30 3.8 Decreased calcium 29 0.5 Increased alkaline phosphatase 28 2.2 Increased triglyceride 26 1.1 Increased glucose 24 1.1 Increased alanine aminotransferase 23 1.6 Hematology Decreased lymphocytes 52 9 Decreased hemoglobin 43 6 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, LOQTORZI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of LOQTORZI in pregnant women. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus and result in fetal death (see Data). Human IgG4 immunoglobulins (IgG4) are known to cross the placenta; therefore, LOQTORZI can potentially be transmitted from the mother to the developing fetus. Advise women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Animal reproduction studies have not been conducted with LOQTORZI to evaluate its effect on reproduction and fetal development. A central function of the PD-1/PD-L1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to the fetus. In murine models of pregnancy, blockade of PD-L1 signaling has been shown to disrupt tolerance to the fetus and to result in an increase in fetal loss; therefore, potential risks of administering LOQTORZI during pregnancy could include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-1/PD-L1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 and PD-L1 knockout mice. Based on its mechanism of action, fetal exposure to toripalimab-tpzi may increase the risk of developing immune-mediated disorders or altering the normal immune response.
8.2 Lactation
Risk Summary
There are no data on the presence of toripalimab-tpzi in human milk or its effects on the breastfed child or on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to toripalimab-tpzi are unknown. Because of the potential for serious adverse reactions in breastfed children, advise lactating women not to breastfeed during treatment with LOQTORZI and for 4 months after the last dose.
8.3 Females and Males of Reproductive Potential
LOQTORZI can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating LOQTORZI [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of LOQTORZI have not been established in pediatric patients [see Indications and Usage (1)].
8.5 Geriatric Use
Of the 146 patients with NPC who were treated with LOQTORZI in combination with cisplatin and gemcitabine 7 (4.8%) were 65 years or older; there were no patients 75 years and older. Clinical studies of LOQTORZI in combination with cisplatin and gemcitabine did not include a sufficient number of patients aged 65 years and over with NPC to determine whether they respond differently from younger patients.
Of the 851 patients with tumor types including nasopharyngeal carcinoma or other types of tumors from the safety pool treated with LOQTORZI as a single agent, 171 (20%) patients were 65 years or older and 13 (1.5%) patients were 75 years and older. No overall differences in safety were observed between elderly patients and younger patients [see Adverse Reactions (6.1)].
Of the 190 patients with NPC treated with LOQTORZI as single agent, 10 (5%) patients were 65 years or older; there were no patients 75 years and older. Clinical studies of LOQTORZI did not include sufficient numbers of patients aged 65 years and over with NPC to determine whether they respond differently from younger patients.
-
11 DESCRIPTION
Toripalimab-tpzi is a programmed death receptor-1 (PD-1) blocking antibody. Toripalimab-tpzi is a humanized immunoglobulin G4 (IgG4) kappa immunoglobulin that has a predicted molecular weight of approximately 150 kDa. It is expressed in a recombinant Chinese Hamster Ovary (CHO) mammalian cell expression system.
LOQTORZI (toripalimab-tpzi) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution for intravenous use supplied in a single-dose vial. Each vial contains 240 mg of LOQTORZI in 6 mL of solution. Each mL of solution contains 40 mg toripalimab-tpzi, citric acid monohydrate (0.51 mg), mannitol (25 mg), polysorbate 80 (0.20 mg), sodium chloride (2.92 mg), sodium citrate (4.65 mg), and Water for Injection, USP, at pH 6.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Toripalimab-tpzi is a humanized IgG4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In syngeneic mouse tumor models, blocking PD-1 activity resulted in decreased tumor growth.
12.2 Pharmacodynamics
The toripalimab-tpzi exposure-response relationships and time course of pharmacodynamic response are not fully characterized.
12.3 Pharmacokinetics
Toripalimab-tpzi pharmacokinetic parameters are presented as geometric mean (coefficient of variation [CV]%) unless otherwise noted. Toripalimab-tpzi concentrations increased in non-linearly over the dose range of 0.3 to 10 mg/kg every two weeks (0.1 to 3.3 times the approved recommended 3 mg/kg dosage in a 64 kg patient). Steady state was reached by Week 7. The mean accumulation ratio was approximately 1.4 for maximum concentration (Cmax) and 1.9 for area under the serum concentration curve (AUC) following multiple doses at the approved recommended dosages of 240 mg Q3W in combination with cisplatin and gemcitabine and 3 mg/kg Q2W as monotherapy.
Distribution
The mean volume of distribution at steady state (Vss) of toripalimab-tpzi was 3.7 L (27%).
Elimination
The mean clearance (CL) was 14.9 mL/h (31%) after the first dose and 9.5 mL/h (36%) at steady state. The mean terminal half-life (t1/2) (± standard deviation) was 10 ± 1.5 days after the first dose and 18 ± 9.4 days at steady state.
Specific Populations
No clinically significant differences in the pharmacokinetics were observed based on age (21 to 85 years), body weight (32 to 164 kg), sex, race (White and Asian), concomitant chemotherapy, mild renal impairment (creatinine clearance [CLcr] 60 to 89 mL/min), mild hepatic impairment (total bilirubin > 1 to 1.5 times ULN with any AST or total bilirubin ≤ ULN with AST > ULN), tumor burden and primary cancer.
The effect of moderate (total bilirubin >1.5 to 3 times ULN and any AST) or severe (total bilirubin > 3 times ULN and any AST) hepatic impairment or of moderate (CLcr 30 to 59 mL/min) or severe (CLcr 15 to 29 mL/min) renal impairment on the pharmacokinetics of toripalimab-tpzi has not been studied.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of LOQTORZI or of other toripalimab products.
Of the 146 evaluable patients in JUPITER-02 with nasopharyngeal cancer who received LOQTORZI 240 mg every 3 weeks for a median duration of 15.1 months, in combination with gemcitabine and cisplatin, 3.4% tested positive for treatment-emergent ADA. Of the 190 evaluable patients in study POLARIS-02 with nasopharyngeal cancer who received LOQTORZI 3 mg/kg every 2 weeks for a median duration of 3.3 months, 3.7% of patients developed treatment-emergent ADA. Neutralizing antibodies have not been tested.
Due to the low incidence of ADA, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, or effectiveness of LOQTORZI is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to test the potential of toripalimab-tpzi for carcinogenicity or genotoxicity.
Fertility studies have not been conducted with toripalimab-tpzi. In 4-week and 26-week repeat-dose toxicology studies in sexually mature cynomolgus monkeys, there were no adverse or notable effects in the male and female reproductive organs.
13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-1/PD-L1 signaling increased the severity of some infections and enhanced inflammatory responses. Mycobacterium tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 blockade using a primate anti-PD1 antibody was also shown to exacerbate M. tuberculosis infection in rhesus macaques. PD-1 and PD-L1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
-
14 CLINICAL STUDIES
14.1 First-line Treatment of Metastatic or Recurrent, Locally Advanced NPC with Cisplatin and Gemcitabine
The efficacy of LOQTORZI in combination with cisplatin and gemcitabine was investigated in JUPITER-02 (NCT03581786), a randomized, multicenter, single region, double-blind, placebo-controlled trial in 289 patients with metastatic or recurrent, locally advanced NPC who had not received previous systemic chemotherapy for recurrent or metastatic disease. Patients with recurrent NPC after treatment with curative intent were required to have an interval of at least 6 months between the last dose of radiotherapy or chemotherapy and recurrence. Patients with autoimmune disease, other than stable hypothyroidism or Type I diabetes, and patients who required systemic immunosuppression were ineligible.
Randomization was stratified according to Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (0 versus 1) and disease stage (recurrent versus metastatic). Patients were randomized (1:1) to receive one of the following treatments:
- LOQTORZI 240 mg intravenously every 3 weeks in combination with cisplatin 80 mg/m2 on Day 1 every 3 weeks gemcitabine 1000 mg/m2 on Days 1 and 8 for up to 6 cycles, followed by LOQTORZI 240 mg once every 3 weeks, or
- Placebo intravenously every 3 weeks in combination with cisplatin 80 mg/m2 on Day 1 every 3 weeks and gemcitabine 1000 mg/m2 on Days 1 and 8 for up to 6 cycles, followed by placebo once every 3 weeks.
Treatment with LOQTORZI or placebo continued until disease progression per RECIST v1.1, unacceptable toxicity, or a maximum of 2 years. Administration of LOQTORZI was permitted beyond radiographic progression if the patient was deriving benefit as assessed by the investigator. Tumor assessments were performed every 6 weeks for the first 12 months and every 9 weeks thereafter. The main efficacy outcome measure was Blinded Independent Review Committee (BIRC)-assessed progression-free survival (PFS) according to RECIST v1.1. Additional efficacy outcome measures include BIRC-assessed overall response rate (ORR) and overall survival (OS).
The study population characteristics were: median age of 48 years (range: 19 to 72), 4.8% age 65 or older, 83% male, 100% Asian, and 57% had ECOG PS of 0. Eighty-six percent of patients had metastatic disease at study entry. Histological subtypes of NPC included 98% non-keratinizing, 1% keratinizing squamous cell carcinoma, and 1% did not have the subtype identified.
Efficacy results of the pre-specified interim analysis of PFS and final analysis of OS are summarized in Table 7 and Figure 1 below. The trial demonstrated statistically significant improvements in BIRC-assessed PFS, ORR and OS for patients randomized to LOQTORZI in combination with cisplatin/gemcitabine compared to cisplatin and gemcitabine with placebo.
Table 7: Efficacy Results in JUPITER-02 Endpoints LOQTORZI
Cisplatin/Gemcitabine
N =146Placebo
Cisplatin/Gemcitabine
N =143BIRC=blinded independent review committee; CI= confidence interval; NR=Not Reached; NE=Not estimable. - * PFS and ORR results are based on the pre-specified interim analysis with data cutoff of May 30, 2020.
- † Based on the stratified Cox proportional-hazards model using the stratification factors at randomization, ECOG performance status and disease stage.
- ‡ Two-sided p-value, based on the stratified log-rank test, as compared with an alpha boundary of 0.010.
- § Two-sided p-value, based upon Cochran-Mantel-Haenszel test.
- ¶ OS results are based on the final analysis with a data cutoff of November 18, 2022.
- # Two-sided p-value, based on the stratified log-rank test, as compared with an alpha boundary of 0.049995.
BIRC-Assessed Progression-free Survival* Number of Events, n (%) 49 (34) 79 (55) Median, months
(95% CI)11.7
(11.0, NE)8.0
(7.0, 9.5)Hazard Ratio (95% CI)† 0.52
(0.36, 0.74)p-value‡ 0.0003 BIRC-Assessed Overall Response Rate* ORR, % (95% CI) 77
(70, 84)66
(58, 74)Complete Response Rate (%) 19 11 Partial Response Rate (%) 58 55 p-value § 0.0353 BIRC-Assessed Duration of Response Median, months
(95% CI)10.0
(8.8, NE)5.7
(5.4, 6.8)Overall Survival¶ Number of Deaths, n (%) 57 (39) 76 (53) Median, months
(95% CI)NR
(38.7, NE)33.7
(27.0, 44.2)Hazard Ratio (95% CI)† 0.63
(0.45, 0.89)p-value# 0.0083 Figure 1 Kaplan-Meier Curves of Overall Survival for JUPITER-02
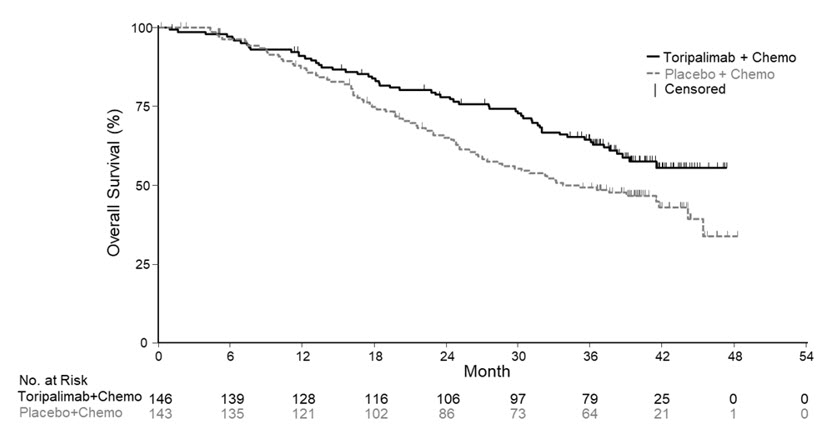
14.2 Previously Treated Unresectable or Metastatic NPC
The efficacy of LOQTORZI was investigated in POLARIS-02 (NCT 02915432), an open-label, multicenter, multicohort trial conducted in a single country. The trial included a total of 172 patients with unresectable or metastatic NPC who had received prior platinum-based chemotherapy for treatment of recurrent or metastatic NPC or had disease progression within 6 months of completion of platinum-based chemotherapy administered as neoadjuvant, adjuvant, or definitive chemoradiation treatment for locally advanced disease. Key exclusion criteria included previous treatment with an anti-PD-(L)1 antibody; active autoimmune disease or other medical conditions requiring immunosuppressive therapy. Patients received LOQTORZI 3 mg/kg intravenously every 2 weeks until disease progression per RECIST v1.1 or unacceptable toxicity. Tumor response assessments were performed every 8 weeks for the first year and every 12 weeks thereafter. The major efficacy outcome measures were confirmed ORR and duration of response (DOR) as assessed by a Blinded Independent Review Committee (BIRC) using RECIST v1.1.
The median age was 45 years (range: 22 to 68), 4.1% age 65 or older, 83% male, 100% Asian, and Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0 (37%). Patients had received a median of 2 prior systemic therapies for recurrent/metastatic disease (range: 1-13). Ninety-five percent of patients had metastatic disease, 95% had non-keratinizing NPC, 2.9% had keratinizing squamous cell carcinoma and 1.7% did not have the subtype identified.
Efficacy results for POLARIS-02 are summarized in Table 8 below.
Table 8: Efficacy Results for POLARIS-02 Endpoint LOQTORZI
(N=172)CI=confidence interval. n=number. NE = not estimable.
BIRC=blinded independent review committee- * Confirmed overall response rate assessed by BIRC
- † Based on observed duration of response
BIRC-Assessed Overall Response Rate* Overall Response Rate, %
(95% CI)21
(15, 28)Complete Response Rate, % 2.3 Partial Response Rate, % 19 BIRC-Assessed Duration of Response (DOR) (N = 36) Median, months
(95% CI)14.9
(10.3, NE)Patients with DOR≥ 6 months†, n (%) 30 (83%) Patients with DOR ≥ 12 months†, n (%) 14 (39%) -
16 HOW SUPPLIED/STORAGE AND HANDLING
LOQTORZI (toripalimab-tpzi), injection is a clear to slightly opalescent, colorless to slightly yellow solution supplied in a carton containing one 240 mg/6 mL (40 mg/mL) single-dose vial (NDC: 70114-340-01).
Store vials refrigerated at 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do not freeze. Do not shake.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Inform patients of the risk of immune-mediated adverse reactions that may be severe or fatal, may occur after discontinuation of treatment, and may require corticosteroid treatment and interruption or discontinuation of LOQTORZI. These reactions may include:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for new or worsening cough, chest pain, or shortness of breath [see Warnings and Precautions (5.1)].
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea or severe abdominal pain [see Warnings and Precautions (5.1)].
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, or easy bruising or bleeding [see Warnings and Precautions (5.1)].
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, or Type 1 diabetes mellitus [see Warnings and Precautions (5.1)].
- Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis [see Warnings and Precautions (5.1)].
- Severe skin reactions: Advise patients to contact their healthcare provider immediately for any signs or symptoms of severe skin reactions, SJS or TEN [see Warnings and Precautions (5.1)].
- Other immune-mediated adverse reactions:
- Advise patients that other immune-mediated adverse reactions can occur and may involve any organ system. Advise patients to contact their healthcare provider immediately for any new or worsening signs or symptoms [see Warnings and Precautions (5.1)].
- Advise patients of the risk of solid organ transplant rejection and to contact their healthcare provider immediately for signs or symptoms of organ transplant rejection [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.2)].
Complications of Allogeneic HSCT
Advise patients of the risk of post-allogeneic hematopoietic stem cell transplantation complications [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
- Advise females of reproductive potential that LOQTORZI can cause harm to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
- Advise females of reproductive potential to use effective contraception during treatment with LOQTORZI and for 4 months after the last dose [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with LOQTORZI and for 4 months after the last dose [see Use in Specific Populations (8.2)].
Laboratory Tests
Advise patients of the importance of keeping scheduled appointments for blood work or other laboratory tests [see Warnings and Precautions (5.1)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Medication Guide
LOQTORZI (lok tor zee)
(toripalimab-tpzi)
injectionThis Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 07/2025 What is the most important information I should know about LOQTORZI?
LOQTORZI is a medicine that may treat nasopharyngeal cancer by working with your immune system. LOQTORZI can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after treatment has ended.
Call or see your healthcare provider right away if you develop any new or worse signs or symptoms, including:
Lung problems- cough
- shortness of breath
- chest pain
Intestinal problems - diarrhea (loose stools) or more frequent bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
- yellowing of skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach-area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
Hormone gland problems - headaches that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increased sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- change in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Kidney problems - decrease in your amount of urine
- blood in your urine
- swelling of your ankles
- loss of appetite
Skin problems - rash
- itching
- skin blistering or peeling
- painful sores or ulcers in your mouth or in your nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with LOQTORZI. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include: - chest pain, irregular heartbeat, shortness of breath, swelling of ankles
- confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- double vision, blurry vision, sensitivity to light, eye pain, changes in eyesight
- persistent or severe muscle pain or weakness, muscle cramps
- low red blood cells, bruising
- chills or shaking
- itching or rash
- flushing
- shortness of breath or wheezing
- dizziness
- feeling like passing out
- fever
- back pain
Rejection of a transplanted organ. Your healthcare provider should tell you what signs and symptoms you should report and monitor, depending on the type of organ transplant that you have had.
Complications, including graft-versus-host disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with LOQTORZI. Your healthcare provider will monitor you for these complications.
Getting medical treatment right away may help keep these problems from becoming more serious.
Your healthcare provider will check you for these problems during treatment with LOQTORZI. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with LOQTORZI if you have severe side effects.What is LOQTORZI?
LOQTORZI is a prescription medicine used to treat adults with a kind of cancer called nasopharyngeal carcinoma (NPC).- LOQTORZI may be used in combination with the chemotherapy medicines cisplatin and gemcitabine, as your first treatment when your NPC has spread to other parts of your body (metastatic) or has returned (recurrent) in nearby tissues (locally advanced).
- LOQTORZI may be used alone to treat your NPC when it:
- has returned and cannot be removed with surgery or
- has spread (metastatic), and
- you received chemotherapy that contains platinum, and it did not work or is no longer working.
Before receiving LOQTORZI, tell your healthcare provider about all of your medical conditions, including if you: - have immune system problems such as Crohn's disease, ulcerative colitis, or lupus
- have received an organ transplant
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have received radiation treatment to your chest area
- have had a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- are pregnant or plan to become pregnant. LOQTORZI can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with LOQTORZI.
- You should use an effective method of birth control during your treatment and for 4 months after your last dose of LOQTORZI. Talk to your healthcare provider about birth control methods that you can use during this time.
- Tell your healthcare provider right away if you think you may be pregnant or if you become pregnant during treatment with LOQTORZI.
- are breastfeeding or plan to breastfeed. It is not known if LOQTORZI passes into your breast milk. Do not breastfeed during treatment and for 4 months after the last dose of LOQTORZI.
How will I receive LOQTORZI? - Your healthcare provider will give you LOQTORZI into your vein through an intravenous (IV) line over 30 or 60 minutes.
- LOQTORZI is usually given every two or three weeks as an intravenous (IV) infusion.
- Your healthcare provider will decide how many treatments you need.
- Your healthcare provider will test your blood to check you for certain side effects.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule.
What are the possible side effects of LOQTORZI?
LOQTORZI can cause serious side effects. See "What is the most important information I should know about LOQTORZI?"
Common side effects of LOQTORZI when used with cisplatin and gemcitabine include:- nausea
- vomiting
- decreased appetite
- constipation
- low levels of thyroid hormone
- rash
- fever
- diarrhea
- burning or feeling of pins and needles in feet and toes
- cough
- muscle and bone pain
- upper respiratory infection
- sleep problems
- dizziness
- feeling generally unwell
Common side effects of LOQTORZI when used alone include: - tiredness
- low levels of thyroid hormone
- muscle and bone pain
These are not all the possible side effects of LOQTORZI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of LOQTORZI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about LOQTORZI that is written for health professionals.What are the ingredients in LOQTORZI?
Active ingredient: toripalimab-tpzi
Inactive ingredients: citric acid monohydrate, mannitol, polysorbate 80, sodium chloride, sodium citrate, and Water for Injection.
Manufactured and Distributed by: Coherus Oncology, Inc. 333 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065, USA
US License No.: 2023
Copyright© 2023 Coherus Oncology, Inc.
All rights reserved.
For more information, call 1-800-483-3692 or go to www.coherus.comPMD-0209, Rev. 01
-
PRINCIPAL DISPLAY PANEL - 240 mg/6 mL Vial Carton
NDC: 70114-340-01
Rx OnlyLOQTORZI™
(toripalimab-tpzi) injection240 mg/6 mL (40 mg/mL)
For Intravenous Infusion After Dilution
ATTENTION PHARMACIST: Each patient is
required to receive the enclosed Medication Guide.1 Single-dose vial. Discard unused portion.
-
INGREDIENTS AND APPEARANCE
LOQTORZI
toripalimab-tpzi injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70114-340 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength toripalimab (UNII: 8JXN261VVA) (toripalimab - UNII:8JXN261VVA) toripalimab 240 mg in 6 mL Product Characteristics Color YELLOW (colorless to slightly yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70114-340-01 1 in 1 CARTON 10/27/2023 1 6 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761240 10/27/2023 Labeler - Coherus Oncology, Inc. (078502849)
Trademark Results [LOQTORZI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LOQTORZI 90642116 not registered Live/Pending |
Coherus BioSciences, Inc. 2021-04-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.