NEORA COMPLEXION CLEARING- salicylic acid swab
Neora by
Drug Labeling and Warnings
Neora by is a Otc medication manufactured, distributed, or labeled by Neora, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
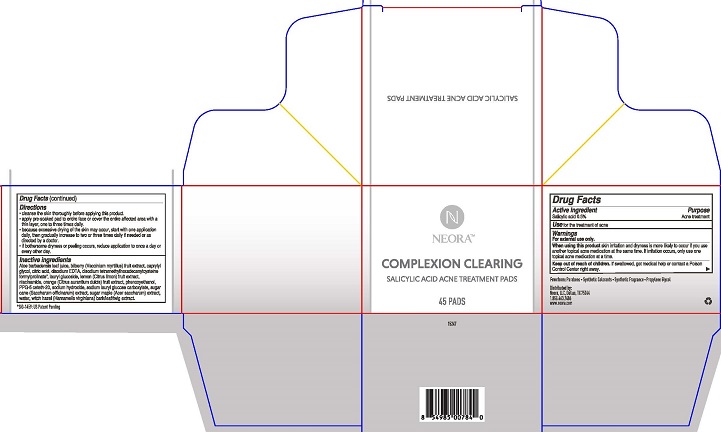
Directions
- cleanse the skin thoroughly before applying this product.
- apply pre-soaked pad to entire face or cover the entire affected area with a thin layer, one to three times daily.
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INACTIVE INGREDIENT
Inactive ingredients Aloe barbadensis leaf juice, bilberry (Vaccinium myrtillus) fruit extract, caprylyl glycol, citric acid, disodium EDTA, disodium tetramethylhexadecenylcysteine formylprolinate, lauryl glucoside, lemon (Citrus limon) fruit extract, niacinamide, orange (Citrus aurantium dulcis) fruit extract, phenoxyethanol, PPG-5 ceteth-20, sodium hydroxide, sodium lauryl glucose carboxylate, sugar cane (Saccharum officinarum) extract, sugar maple (Acer saccharum) extract, water, witch hazel (Hamamelis virginiana) bark/leaf/twig extract.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEORA COMPLEXION CLEARING
salicylic acid swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72935-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength BILBERRY (UNII: 9P2U39H18W) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) CITRUS LIMON FRUIT OIL (UNII: 0HNC1J1YED) NIACINAMIDE (UNII: 25X51I8RD4) CITRUS AURANTIUM FRUIT OIL (UNII: 59JDQ5VT0T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PPG-5-CETETH-20 (UNII: 4AAN25P8P4) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL GLYCOL CARBOXYLATE (UNII: 8L0472VMYL) SACCHARUM OFFICINARUM WHOLE (UNII: 3Z20C92XNB) ACER SACCHARUM SAP (UNII: 75UOH57984) WATER (UNII: 059QF0KO0R) WITCH HAZEL (UNII: 101I4J0U34) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72935-001-45 1 in 1 CARTON 05/01/2019 1 45 in 1 JAR 1 1.1 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 05/01/2019 Labeler - Neora, LLC (055050917)
Trademark Results [Neora]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEORA 90081973 not registered Live/Pending |
Jin Changde 2020-07-29 |
 NEORA 87983731 not registered Live/Pending |
NEORA SWITZERLAND HOLDINGS GMBH 2018-02-13 |
 NEORA 87796251 not registered Live/Pending |
NERIUM INTERNATIONAL SWITZERLAND HOLDING 2018-02-13 |
 NEORA 86107794 not registered Dead/Abandoned |
CGTN C.V. 2013-11-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.