ZO MEDICAL SURFATROL ASTRINGENT- aluminum acetate powder
ZO MEDICAL SURFATROL Astringent by
Drug Labeling and Warnings
ZO MEDICAL SURFATROL Astringent by is a Otc medication manufactured, distributed, or labeled by ZO Skin Health, Inc., Paramount Cosmetics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients Purpose - * When combined together in water, these ingredients form the active ingredient aluminum acetate. See Directions
Aluminum Sulfate Tetradecahydrate, 2850 mg Astringent* Calcium Acetate Monohydrate, 2016 mg Astringent* - Uses
- Warnings
-
Directions
- dissolve 1 packet in 12 oz. of cool or warm water.
- stir until fully dissolved; do not strain or filter. The resulting mixture contains 0.45% aluminum acetate and is ready for use.
For use as a compress or wet dressing:
- dip clean gauze in the solution
- apply gauze loosely to affected area for 1 to 2 minutes
- repeat 3-4 times a day as needed or as directed by a doctor
- discard solution after each use
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
-
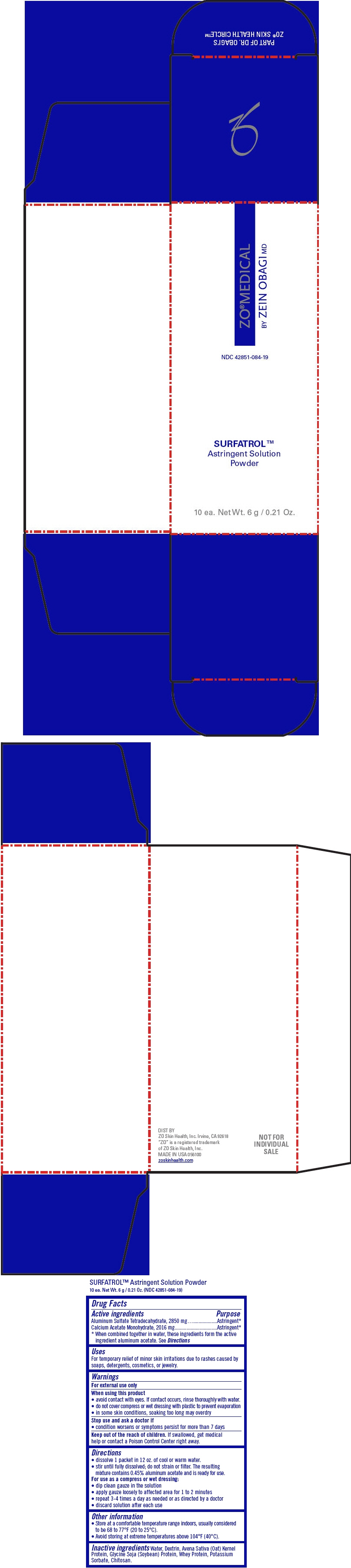
PRINCIPAL DISPLAY PANEL - 6 g Packet Carton
ZO®MEDICAL
BY ZEIN OBAGI MDNDC: 42851-084-19
SURFATROL™
Astringent Solution
Powder10 ea. Net Wt. 6 g / 0.21 Oz.
-
INGREDIENTS AND APPEARANCE
ZO MEDICAL SURFATROL ASTRINGENT
aluminum acetate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42851-084 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Aluminum Acetate (UNII: 80EHD8I43D) (Aluminum Cation - UNII:3XHB1D032B) Aluminum Acetate 4866 mg in 4.866 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Whey (UNII: 8617Z5FMF6) Potassium Sorbate (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42851-084-19 10 in 1 CARTON 08/01/2016 1 6 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 08/01/2016 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations Paramount Cosmetics 001321058 MANUFACTURE(42851-084)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.