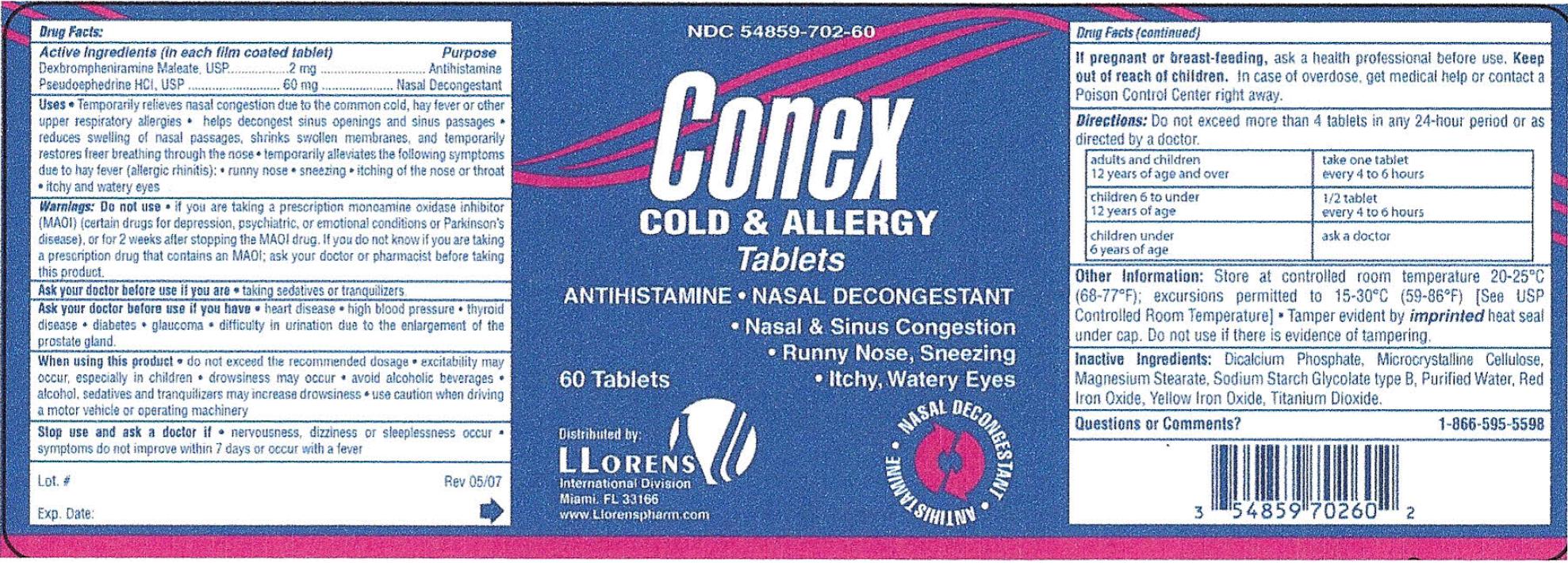
CONEX- dexbrompheniramine maleate, pseudoephedrine hcl tablet
Conex by
Drug Labeling and Warnings
Conex by is a Otc medication manufactured, distributed, or labeled by LLORENS PHARMACEUTICALS INTERNATIONAL DIVISION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
PURPOSE
Uses
- Temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- helps decongest sinus openings and sinus passages
- Reduces swelling of nasal passages, shrinks swollen membranes, and temporarily restores freer breathing through the nose
- Temporarily alleviates the following symptoms due to hay fever (allergic rhinitis): runny nose, sneezing, itching of the nose or throat, itching and watery eyes.
-
WARNINGS
Warnings:
Ask a doctor before you use if you are
- taking sedatives or tranquilizers.
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- difficulty in urination due to the enlargement of the prostate gland.
- do not exceed the recommended dosage
- excitability may occur, especially in children
- drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- nervousness, dizziness or sleepiness occur
- symptoms do not improve withing 7 days or occur with a fever
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
-
DO NOT USE
Do not use if you are taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions
or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if you are taking a prescription drug that contains MAOI;
ask your doctor or pharmacist before taking this product.
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Other Information: Store at controlled room temperature 20 - 25 degree celsius (68 - 77 degree fahrenheit); excursions permitted to
15 - 30 degree celsius ( 59 - 86 degree fahrenheit) [ See USP Controlled Room Temperature] Tamper evident by imprinted heat seal
under cap. Do not use if there is evidence of tampering.
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CONEX
dexbrompheniramine maleate, pseudoephedrine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54859-702 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXBROMPHENIRAMINE MALEATE (UNII: BPA9UT29BS) (DEXBROMPHENIRAMINE - UNII:75T64B71RP) DEXBROMPHENIRAMINE MALEATE 2 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE B POTATO (UNII: 27NA468985) WATER (UNII: 059QF0KO0R) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape OVAL Size 13mm Flavor Imprint Code LLORENS Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54859-702-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 11/01/2007 Labeler - LLORENS PHARMACEUTICALS INTERNATIONAL DIVISION (037342305) Registrant - LLORENS PHARMACEUTICALS INTERNATIONAL DIVISION (037342305)
Trademark Results [Conex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CONEX 90038190 not registered Live/Pending |
Trilogy Networks Inc 2020-07-06 |
 CONEX 88210793 5787638 Live/Registered |
CV Technology, Inc. 2018-11-29 |
 CONEX 87564850 not registered Dead/Abandoned |
Aziyo Med, LLC 2017-08-11 |
 CONEX 86373655 4806849 Live/Registered |
American Maplan Corporation 2014-08-21 |
 CONEX 85690105 not registered Dead/Abandoned |
Pharmaceutical Generic Developers Inc. USA 2012-07-30 |
 CONEX 85542294 not registered Dead/Abandoned |
Advanced Expos LLC 2012-02-14 |
 CONEX 85126705 4107494 Live/Registered |
CONEX UNIVERSAL LIMITED 2010-09-10 |
 CONEX 79159108 4910798 Live/Registered |
Conex IPR Limited 2014-11-14 |
 CONEX 78187847 2831935 Live/Registered |
THE EUCLID CHEMICAL COMPANY 2002-11-22 |
 CONEX 76402597 2683447 Dead/Cancelled |
Norwolf Tool Works 2002-04-29 |
 CONEX 76313726 2707843 Dead/Cancelled |
CONEX, L.L.C. 2001-09-19 |
 CONEX 76156217 not registered Dead/Abandoned |
Key Productions, Inc. 2000-10-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.