CETIRIZINE HYDROCHLORIDE tablet, film coated
CETIRIZINE HYDROCHLORIDE by
Drug Labeling and Warnings
CETIRIZINE HYDROCHLORIDE by is a Otc medication manufactured, distributed, or labeled by Bionpharma Inc., OrBion Pharmaceuticals Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
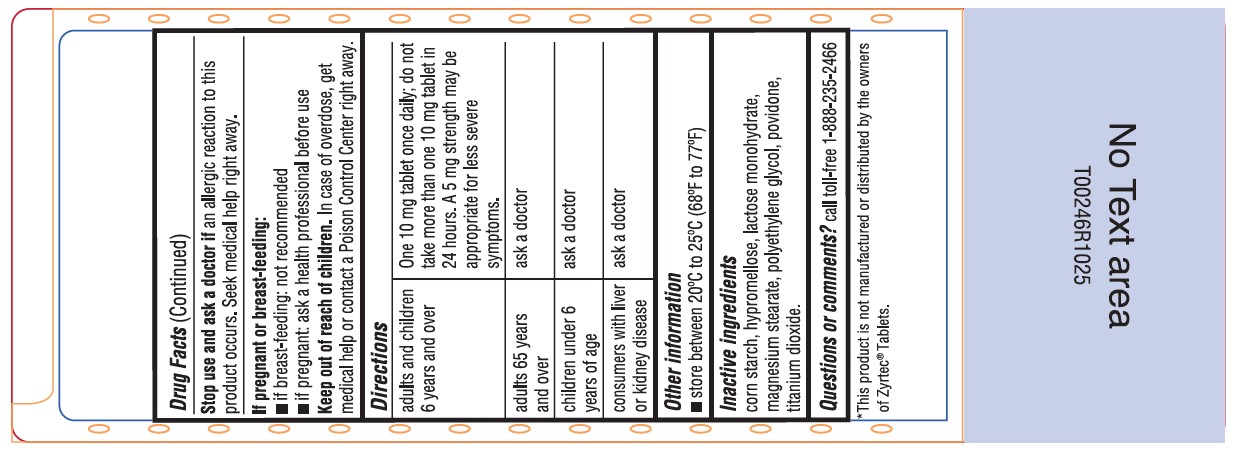
Warnings
Do not useif you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you haveliver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery.
Stop use and ask a doctor ifan allergic reaction to this product occurs. Seek medical help right away.
-
Directions
adults and children 6 years and over one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours.
A 5 mg product may be appropriate for less severe symptoms.adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69452-465 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE K29/32 (UNII: 390RMW2PEQ) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white (white to off-white) Score no score Shape CAPSULE (rounded off rectangular) Size 9mm Flavor Imprint Code S;521 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69452-465-81 400 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078862 10/20/2024 Labeler - Bionpharma Inc. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations OrBion Pharmaceuticals Private Limited 854403569 manufacture(69452-465) , analysis(69452-465) , pack(69452-465) , label(69452-465)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.