WALGREENS NERVE PAIN RELIEVING TOPICAL ANALGESIC- lidocaine hydrochloride and menthol, unspecified form liquid
Walgreens Nerve Pain Relieving by
Drug Labeling and Warnings
Walgreens Nerve Pain Relieving by is a Otc medication manufactured, distributed, or labeled by Walgreen Company, Garcoa, Inc., Sigan Industries INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- ACTIVE INGREDIENT
- Use
-
Warnings
For external use only
Do not use
- in large quantities, particularly over raw surfaces or blistered areas
- if you are allergic to lidocaine or other local anesthetics
- on damaged, broken skin
- on cuts, swollen or irritated skin
- for more than a week without consulting a physician.
When using this product
- use only as directed
- avoid contact with the eyes
- do not use with heating pads, medicated patches or heating devices
- do not bandage tightly
- read and follow all directions and warnings on this carton
- avoid applying into skin folds
- do not apply over large areas of the body
- a slight burning sensation may occur upon application but usually disappears in several days.
-
Directions
Adults and children 12 years of age and older
- clean and dry affected area
- apply a thin layer to affected area not more than 3 times daily
- do not apply more than three times within 24 hours
- massage into painful area until thoroughly absorbed into the skin
- wash hands after use with soap and water.
Children under 12 years of age: consult a doctor.
- Other information
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, alcohol denat., aminomethyl propanol, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, isohexadecane, phenoxyethanol, polysorbate 60, steareth-21, water.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 74 mL Cylinder Carton
Walgreens
Compare to the active ingredients in
Nervive® Roll-On Pain Relieving Liquid††WALGREENS
PHARMACIST RECOMMENDED†Nerve Pain
Relieving
LiquidLIDOCAINE HCl 4% / TOPICAL ANALGESIC /
MENTHOL 1% / TOPICAL ANALGESICRoll-On
- Maximum strength
lidocaine - Helps to penetrate
for targeted, numbing
pain relief - Easy to apply to affected
area with roll-on
applicator
NOT ACTUAL SIZE
2.5 FL OZ (74 mL)
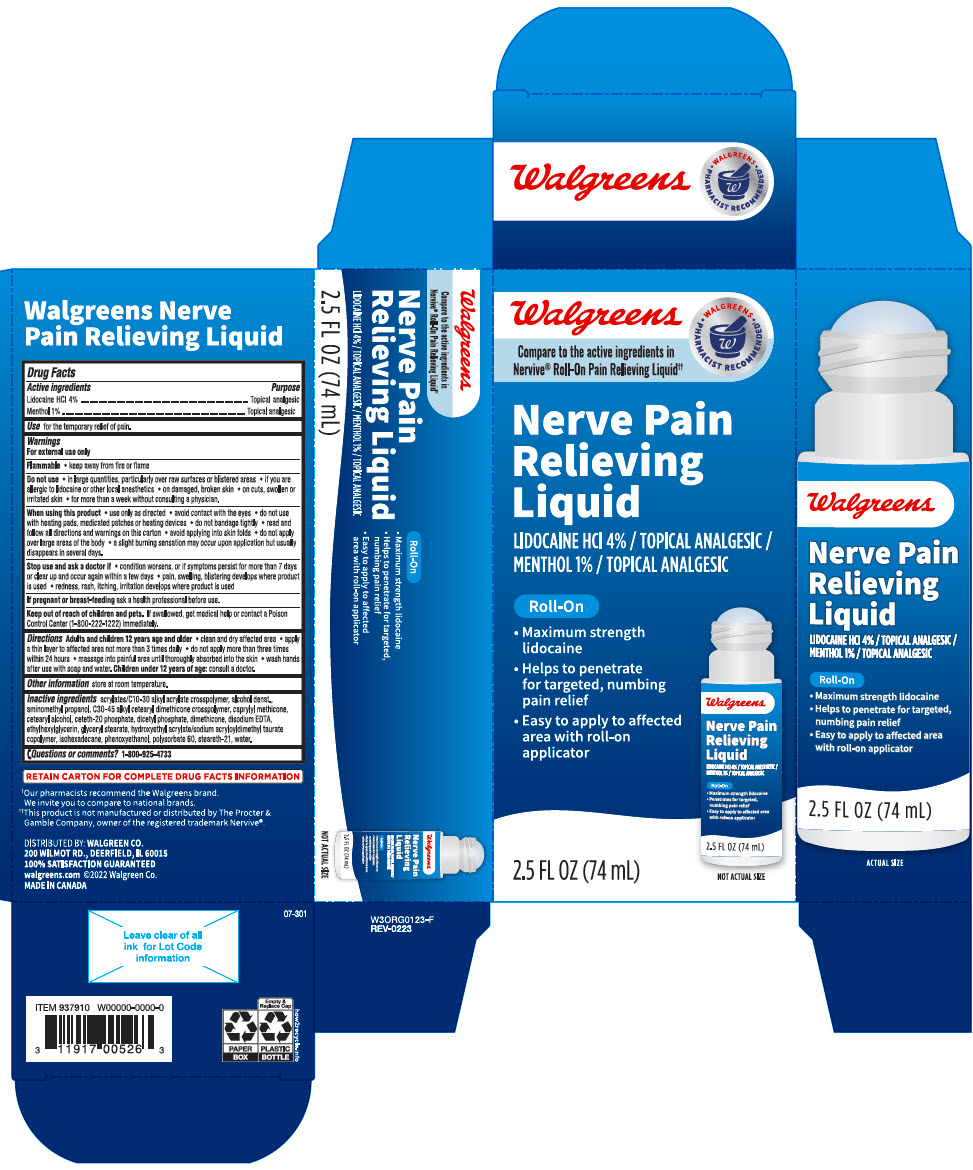
- Maximum strength
-
INGREDIENTS AND APPEARANCE
WALGREENS NERVE PAIN RELIEVING TOPICAL ANALGESIC
lidocaine hydrochloride and menthol, unspecified form liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0363-3791 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine Hydrochloride (UNII: V13007Z41A) (Lidocaine - UNII:98PI200987) Lidocaine Hydrochloride Anhydrous 40 mg in 1 mL Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Alcohol (UNII: 3K9958V90M) Aminomethylpropanol (UNII: LU49E6626Q) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (UNII: 4ZK9VP326R) Caprylyl Trisiloxane (UNII: Q95M2P1KJL) Cetostearyl Alcohol (UNII: 2DMT128M1S) Ceteth-20 Phosphate (UNII: 921FTA1500) Dihexadecyl Phosphate (UNII: 2V6E5WN99N) Dimethicone (UNII: 92RU3N3Y1O) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Ethylhexylglycerin (UNII: 147D247K3P) Glyceryl Monostearate (UNII: 230OU9XXE4) Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) Isohexadecane (UNII: 918X1OUF1E) Phenoxyethanol (UNII: HIE492ZZ3T) Polysorbate 60 (UNII: CAL22UVI4M) Steareth-21 (UNII: 53J3F32P58) Water (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0363-3791-01 1 in 1 CARTON 02/21/2023 1 74 mL in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/21/2023 Labeler - Walgreen Company (008965063) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations SV Labs Toronto Corporation 203917476 manufacture(0363-3791)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.