EGRIFTA WR- tesamorelin kit
EGRIFTA WR by
Drug Labeling and Warnings
EGRIFTA WR by is a Prescription medication manufactured, distributed, or labeled by Theratechnologies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EGRIFTA WR safely and effectively. See full prescribing information for EGRIFTA WR.
EGRIFTA WR™ (tesamorelin) for injection, for subcutaneous use
Initial U.S. Approval: 2010RECENT MAJOR CHANGES
Dosage and Administration (2)
03/2025
INDICATIONS AND USAGE
EGRIFTA WR is a growth hormone-releasing factor (GHRF) analog indicated for the reduction of excess abdominal fat in HIV-infected adult patients with lipodystrophy. (1)
Limitations of use:
DOSAGE AND ADMINISTRATION
- The recommendations in this prescribing information only apply to EGRIFTA WR (tesamorelin) for injection 11.6 mg per vial formulation. For recommendations for tesamorelin for injection 2 mg per vial formulation, see the EGRIFTA SV prescribing information. These two formulations and strengths have differences in the dosage, the number of vials required to prepare a dose, reconstitution instructions, and storage requirements. EGRIFTA WR and EGRIFTA SV are not substitutable. (2.1).
- The dose of EGRIFTA WR is 1.28 mg (0.16 mL of the reconstituted solution) injected subcutaneously once daily. (2.1)
- Inject EGRIFTA WR into the abdomen, rotating injection sites. (2.1,5.6)
- Use only the diluent provided, Bacteriostatic Water for Injection, USP, to reconstitute EGRIFTA WR. (2.2)
- Reconstitute one vial of lyophilized powder with 1.3 mL of diluent. Move the vial in a circle (swirl) to mix all the powder and liquid. Do not shake. (2.2)
- Inspect the reconstituted vial visually for particulate matter and discoloration. Use only if the solution is clear, colorless and without particulate matter. (2.2)
- One reconstituted vial provides daily doses for 7 days. Discard unused solution of EGRIFTA WR vial 7 days after mixing. (2.2)
DOSAGE FORMS AND STRENGTHS
- For injection: 11.6 mg of tesamorelin as a lyophilized powder in single-patient-use vial for reconstitution. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Increased risk of neoplasms: Preexisting malignancy should be inactive and its treatment complete prior to starting EGRIFTA WR. Discontinue EGRIFTA WR if there is any evidence of recurrent malignancy. (5.1)
- Elevated IGF-1: EGRIFTA WR stimulates GH production and increases serum IGF-1, a growth factor. The effects of prolonged elevations in IGF-1 levels are unknown. Monitor IGF-1 levels during EGRIFTA WR therapy. Consider discontinuing in patients with persistent elevations. (5.2)
- Fluid retention: May occur with EGRIFTA WR and may include edema, arthralgia, and carpal tunnel syndrome. (5.3)
- Glucose intolerance or diabetes mellitus: May develop with EGRIFTA WR use. Evaluate glucose prior to and during therapy. (5.4)
- Hypersensitivity reactions: Have occurred in clinical trials. Advise patients to seek immediate medical attention and discontinue treatment if suspected. (5.5)
- Increased mortality in patients with acute critical illness: Consider discontinuation in critically ill patients. (5.7)
ADVERSE REACTIONS
Most commonly reported adverse reactions (>5%): Arthralgia, injection site erythema, injection site pruritus, pain in extremity, peripheral edema, and myalgia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact THERA patient support® toll free at 1-833-23THERA (1-833-238-4372) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Cytochrome P450-metabolized drugs: Monitor patients for potential interactions when administering with EGRIFTA WR. (7.1)
- Glucocorticoids: Patients receiving glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in maintenance or stress doses following initiation of EGRIFTA WR. (7.2)
USE IN SPECIFIC POPULATIONS
- Lactation: HIV-1 infected mothers should not breastfeed to avoid potential postnatal transmission of HIV-1. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration Instructions
2.2 Reconstitution Procedure
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Neoplasms
5.2 Elevated IGF-1 Levels
5.3 Fluid Retention
5.4 Glucose Intolerance or Diabetes Mellitus
5.5 Hypersensitivity Reactions
5.6 Injection Site Reactions
5.7 Increased Mortality in Patients with Acute Critical Illness
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Metabolized Drugs
7.2 Glucocorticoids
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
EGRIFTA WR is indicated for the reduction of excess abdominal fat in HIV-infected adult patients with lipodystrophy.
Limitations of Use:
- Long-term cardiovascular safety of EGRIFTA WR has not been established. Consider risk/benefit of continuation of treatment in patients who have not had a reduction in visceral adipose tissue.
- EGRIFTA WR is not indicated for weight loss management as it has a weight neutral effect.
- There are no data to support improved compliance with anti-retroviral therapies in HIV-positive patients taking EGRIFTA WR.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration Instructions
- There are two EGRIFTA formulations (EGRIFTA WR and EGRIFTA SV) with different recommended dosages. These two formulations and strengths have differences in the dosage, the number of vials required to prepare a dose, reconstitution instructions, and storage requirements.
- EGRIFTA WR and EGRIFTA SV are not substitutable.
- The dosage and administration recommendations in this prescribing information only apply to EGRIFTA WR (tesamorelin) for injection 11.6 mg per vial formulation. For dosage and administration recommendations for tesamorelin for injection 2 mg per vial formulation, see the EGRIFTA SV prescribing information.
- The recommended dosage of EGRIFTA WR is 1.28 mg subcutaneously once daily.
- Inject EGRIFTA WR into the abdomen. Rotate injection sites to different areas of the abdomen [see Warnings and Precautions (5.6)]. Do not inject into scar tissue, bruises or the navel.
2.2 Reconstitution Procedure
- Instruct patients to read the Instructions for Use enclosed in the EGRIFTA WR Injection Box.
- Use only the diluent provided, Bacteriostatic Water for Injection, to reconstitute EGRIFTA WR.
- Reconstitute 1 vial of EGRIFTA WR lyophilized powder with 1.3 mL of diluent (8 mg per mL). Move the vial in a circle (swirl) to mix all the powder and liquid. Do not shake.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. EGRIFTA WR is a clear, colorless solution. Do not use if solid particles appear or if the solution is cloudy or colored.
- One reconstituted EGRIFTA WR vial provides daily doses for 7 consecutive days. One dose of EGRIFTA WR is 1.28 mg in 0.16 mL of the reconstituted solution.
- Store reconstituted EGRIFTA WR at room temperature at 20°C to 25°C (68°F to 77°F). Discard unused solution of EGRIFTA WR 7 days after mixing. Do not freeze.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
EGRIFTA WR is contraindicated in:
- Patients with disruption of the hypothalamic-pituitary axis due to hypophysectomy, hypopituitarism, pituitary tumor/surgery, head irradiation or head trauma.
- Patients with active malignancy. Any preexisting malignancy should be inactive and its treatment complete prior to instituting therapy [see Warnings and Precautions (5.1)].
- Patients with known hypersensitivity to tesamorelin or the excipients in EGRIFTA WR [see Warnings and Precautions (5.5)].
- Pregnant women because modifying visceral adipose tissue offers no benefit in a pregnant woman and could result in fetal harm [see Use in Specific Populations (8.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Neoplasms
New Malignancy
Carefully consider the decision to start treatment with EGRIFTA WR based on the increased background risk of malignancies in HIV-positive patients.
Active Malignancy
EGRIFTA WR induces the release of endogenous growth hormone (GH), a known growth factor. Do not treat patients with active malignancy with EGRIFTA WR [see Contraindications (4)].
History of Malignancy
For patients with a history of non-malignant neoplasms, initiate EGRIFTA WR therapy after careful evaluation of the potential benefit of treatment. For patients with a history of treated and stable malignancies, initiate EGRIFTA WR therapy only after careful evaluation of the potential benefit of treatment relative to the risk of re-activation of the underlying malignancy. Discontinue EGRIFTA WR if there is any evidence of recurrent malignancy.
5.2 Elevated IGF-1 Levels
EGRIFTA WR stimulates GH production and increases serum IGF-1, a growth factor. The effects of prolonged elevations in IGF-1 levels are unknown. Monitor IGF-1 levels during EGRIFTA WR therapy. Consider discontinuing EGRIFTA WR in patients with persistent elevations of IGF-1 levels (e.g., >3 SDS), particularly if the efficacy response is not robust.
Among patients who received EGRIFTA for 26 weeks, 47% had IGF-1 levels greater than 2 standard deviation scores (SDS), and 36% had SDS >3, with this effect seen as early as 13 weeks of treatment. Among those patients who remained on EGRIFTA for a total of 52 weeks, at the end of treatment, 34% had IGF-1 SDS >2 and 23% had IGF-1 SDS >3.
5.3 Fluid Retention
Fluid retention may occur during EGRIFTA WR therapy and is thought to be related to the induction of GH secretion. This manifests as increased tissue turgor and musculoskeletal discomfort resulting in adverse reactions (e.g. edema, arthralgia, and carpal tunnel syndrome) which are either transient or resolve with discontinuation of treatment.
5.4 Glucose Intolerance or Diabetes Mellitus
EGRIFTA WR treatment can result in glucose intolerance. During clinical trials, the percentages of patients with elevated HbA1c (≥ 6.5%) from baseline to Week 26 were 5% and 1% in the EGRIFTA and placebo groups, respectively. An increased risk of developing diabetes with EGRIFTA (HbA1c level ≥ 6.5%) relative to placebo was observed [intent-to-treat hazard odds ratio of 3.3 (CI 1.4, 9.6)].
Evaluate glucose status prior to initiating EGRIFTA WR. Monitor all patients treated with EGRIFTA WR periodically to diagnose those who develop impaired glucose tolerance or diabetes. If patients treated with EGRIFTA WR develop glucose intolerance or diabetes, consider discontinuing EGRIFTA WR in patients who do not show a clear efficacy response.
EGRIFTA WR increases IGF-1, monitor patients with diabetes who are receiving treatment with TESAMORELIN for injection at regular intervals for potential development or worsening of retinopathy.
5.5 Hypersensitivity Reactions
Hypersensitivity reactions occurred in 4% of patients treated with EGRIFTA in clinical trials. Reactions included pruritus, erythema, flushing, urticaria, and rash. In cases of suspected hypersensitivity reactions, advise patients to seek prompt medical attention and immediately discontinue treatment with EGRIFTA WR.
5.6 Injection Site Reactions
EGRIFTA WR treatment may cause injection site reactions, including injection site erythema, pruritus, pain, irritation, and bruising. The incidence of injection site reactions was 25% in EGRIFTA treated patients and 14% in placebo-treated patients during the first 26 weeks of treatment in clinical trials. Rotate injection sites to different areas of the abdomen to decrease injection site reactions [see Dosage and Administration (2.1)].
5.7 Increased Mortality in Patients with Acute Critical Illness
Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported after treatment with pharmacologic amounts of growth hormone. EGRIFTA WR is a growth hormone-releasing hormone (GHRH) and since it stimulates growth hormone production, consider discontinuing EGRIFTA WR in critically ill patients.
-
6 ADVERSE REACTIONS
The following important adverse reactions are also described elsewhere in the labeling:
- Increased risk of neoplasms [see Warnings and Precautions (5.1)]
- Elevated IGF-1 levels [see Warnings and Precautions (5.2)]
- Fluid retention [see Warnings and Precautions (5.3)]
- Glucose intolerance or diabetes mellitus [see Warnings and Precautions (5.4)]
- Hypersensitivity reactions [see Warnings and Precautions (5.5)]
- Injection site reactions [see Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of EGRIFTA WR (11.6 mg/vial formulation) has been established based on clinical trials conducted with EGRIFTA (1 mg/vial formulation). Adverse reactions for the 1.28 mg dose (11.6 mg/vial formulation) of EGRIFTA WR are expected to be similar to those observed with the 2 mg dose (1 mg/vial formulation) of EGRIFTA [see Clinical Pharmacology (12.3)].
Seven hundred and forty (740) HIV-infected patients with lipodystrophy and excess abdominal fat were treated with EGRIFTA in clinical trials; of these, 543 received EGRIFTA during the initial 26-week placebo-controlled phase.
The most commonly reported adverse reactions were hypersensitivity reactions (e.g., rash, urticaria), edema-related reactions (e.g., arthralgia, extremity pain, peripheral edema, and carpal tunnel syndrome), hyperglycemia, and injection site reactions (injection site erythema, pruritus, pain, urticaria, irritation, swelling, and hemorrhage).
Adverse reactions that occurred more frequently with EGRIFTA relative to placebo and had an incidence ≥1% during the first 26 weeks across all studies are presented in Table 1.
Table 1. Adverse Reactions Reported in ≥ 1% and More Frequent in EGRIFTA–treated than Placebo Patients during the 26-Week Phase (Combined Studies) * Injection site reaction includes: Injection site erythema, Injection site pruritus, Injection site rash, Injection site urticaria, Injection site pain, Injection site swelling, Injection site irritation, Injection site hemorrhage.
Preferred Term Placebo
(N=263)EGRIFTA
(N=543)Injection site reaction*
Arthralgia
Pain in extremity
Myalgia
Edema peripheral
Paresthesia
Hypoesthesia
Rash
Dyspepsia
Musculoskeletal pain
Pain
Pruritus
Vomiting
Musculoskeletal stiffness
Blood creatine phosphokinase increased
Carpal tunnel syndrome
Joint swelling
Muscle strain
Night sweats
Palpitations6
11
5
2
2
2
2
2
1
1
1
1
0
0
0
0
0
0
0
017
13
6
6
6
5
4
4
2
2
2
2
3
2
1
1
1
1
1
1In the EGRIFTA clinical trials, mean baseline HbA1c was 5.3% among patients in both the EGRIFTA and placebo groups. Patients receiving EGRIFTA had an increased risk of developing diabetes (HbA1c level ≥ 6.5%) compared with placebo (5% vs. 1%), with a hazard ratio of 3.3 (CI 1.4, 9.6).
-
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Metabolized Drugs
Co-administration of tesamorelin with simvastatin, a CYP3A substrate had no significant impact on the pharmacokinetics profiles of simvastatin in healthy subjects [see Clinical Pharmacology (12.3)].
EGRIFTA WR stimulates GH production. Published data indicate that GH may modulate cytochrome P450 (CYP450) mediated antipyrine clearance. These data suggest that GH may alter the clearance of compounds known to be metabolized by CYP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, and cyclosporine). Monitor patients for potential interactions when administering EGRIFTA WR in combination with other drugs known to be metabolized by CYP450 liver enzymes.
7.2 Glucocorticoids
GH inhibits 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1), a microsomal enzyme required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. EGRIFTA WR stimulates GH production; therefore, patients receiving glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in maintenance or stress doses following initiation of EGRIFTA WR. Patients treated with cortisone acetate and prednisone may be affected more than others because conversion of these drugs to their biologically active metabolites is dependent on the activity of 11βHSD-1.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
EGRIFTA WR is contraindicated in pregnant women because modifying visceral adipose tissue offers no benefit in pregnant women and could result in fetal harm [see Clinical Considerations and Contraindications (4)]. Administration of tesamorelin acetate to rats during organogenesis resulted in hydrocephaly in offspring at a dose of approximately two and four times the clinical dose, based on measured drug exposure (AUC). If EGRIFTA WR is used during pregnancy, or if the patient becomes pregnant while taking it, discontinue EGRIFTA WR.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
During pregnancy, visceral adipose tissue increases due to normal metabolic and hormonal changes. Modifying pregnancy-associated physiologic changes in visceral adipose tissue with EGRIFTA WR offers no known benefit and could result in fetal harm.
Data
Animal Data
Tesamorelin acetate administration to rats during organogenesis and lactation resulted in hydrocephaly in offspring at a dose of approximately two and four times the clinical dose, respectively, based on measured drug exposure (AUC). Actual animal dose was 1.2 mg/kg. During organogenesis, lower doses approximately 0.1 to 1-times the clinical dose caused delayed skull ossification in rats. Actual animal doses were 0.1 to 0.6 mg/kg. No adverse developmental effects occurred in rabbits using doses up to approximately 500 times the clinical dose.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection. There are no data on the presence of tesamorelin in human milk, the effects on the breastfed child, or the effects on milk production. Because of both the potential for (1) HIV-1 infection transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive patients), and (3) any possible adverse effects of tesamorelin, mothers should not breastfeed if they receive EGRIFTA WR.
8.4 Pediatric Use
The safety and effectiveness of EGRIFTA WR in pediatric patients have not been established.
In pediatric patients with open epiphyses, treatment with EGRIFTA WR may result in linear growth acceleration and excessive growth. EGRIFTA WR is not indicated for use in pediatric patients with open or closed epiphyses.
-
11 DESCRIPTION
Tesamorelin is a human growth hormone-releasing factor (GRF) analog produced synthetically. It is comprised of the 44 amino acid sequence of human GRF and a hexenoyl moiety, a C6 chain with a double bond at position 3, attached to the tyrosine residue at the N-terminal part of the molecule. Tesamorelin is prepared as an acetate salt. The molecular formula of tesamorelin acetate is C221H366N72O67S x C2H4O2 (x ≈ 7) and its molecular weight (as free base equivalent) is 5135.9 Da. The structural formula of tesamorelin acetate is:

EGRIFTA WR (tesamorelin) for injection is a sterile, white to off-white, preservative-free lyophilized powder for subcutaneous injection. Each single-patient-use vial of EGRIFTA WR contains tesamorelin 11.6 mg (equivalent to approximately 11.9 mg of tesamorelin acetate) and the following inactive ingredients: 145 mg hydroxypropyl betadex, 43.5 mg mannitol. Hydrochloric acid and/or sodium hydroxide may be used to adjust the pH. The pH of EGRIFTA WR is between 4.5 and 7.4. After reconstitution with 1.3 mL of Bacteriostatic Water for Injection, USP, resultant concentration is 8 mg/mL and the solution is clear and colorless. Bacteriostatic Water for Injection, USP contains benzyl alcohol as preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In vitro, tesamorelin binds and stimulates human GRF receptors with similar potency as the endogenous GRF [see Clinical Pharmacology (12.2)].
Growth hormone-releasing factor (GHRF), also known as growth hormone-releasing hormone (GHRH), is a hypothalamic peptide that acts on the pituitary somatotroph cells to stimulate the synthesis and pulsatile release of endogenous growth hormone (GH), which is both anabolic and lipolytic. GH exerts its effects by interacting with specific receptors on a variety of target cells, including chondrocytes, osteoblasts, myocytes, hepatocytes, and adipocytes, resulting in a host of pharmacodynamic effects. Some, but not all these effects, are primarily mediated by IGF-1 produced in the liver and in peripheral tissues.
12.2 Pharmacodynamics
Tesamorelin stimulates growth hormone secretion, and subsequently increases IGF-1 and IGFBP-3 levels. No clinically significant changes in the levels of other pituitary hormones, including thyroid-stimulating hormone (TSH), luteinizing hormone (LH), adrenocorticotropic hormone (ACTH) and prolactin, were observed in patients receiving EGRIFTA in clinical trials.
12.3 Pharmacokinetics
Absorption
The absolute bioavailability of tesamorelin after subcutaneous administration of a 2 mg dose of EGRIFTA (1 mg/vial formulation) was determined to be less than 4% in healthy adult subjects.
Single and multiple dose pharmacokinetics have been characterized in healthy subjects and HIV-infected patients without lipodystrophy using a 2 mg dose of EGRIFTA (1 mg/vial formulation). Tesamorelin mean extent of absorption (AUC) was 34% higher in HIV-infected patients than healthy subjects. Tesamorelin peak plasma concentration (Cmax) was similar in HIV-infected patients and healthy subjects. The median peak plasma tesamorelin concentration (Tmax) was 0.15 h in both populations.
Following single dose of subcutaneous administration of 1.28 mg of EGRIFTA WR (11.6 mg/vial formulation) in healthy subjects, the mean [coefficient of variation (CV)] AUC0-inf was 1172 (48%) pg.h/mL. The mean (CV) Cmax value was 3831 (40%) pg/mL and the median Tmax was 0.15 h.
The systemic exposure (Cmax and AUCs) of tesamorelin is similar between the 1.28 mg dose of EGRIFTA WR (11.6 mg/vial formulation) and the 2 mg dose of EGRIFTA (1 mg/vial formulation).
Distribution
The mean volume of distribution (±SD) of tesamorelin following a single subcutaneous administration of the 1.28 mg dose of EGRIFTA WR (11.6 mg/vial formulation) was 4.8 ± 1.9 L/kg in healthy subjects.
Elimination
Mean elimination half-life (t1/2) of tesamorelin was 11 minutes in healthy subjects after single dose subcutaneous administration of the 1.28 mg of EGRIFTA WR (11.6 mg/vial formulation).
Specific Populations
Pharmacokinetics of tesamorelin in patients with renal or hepatic impairment, in pediatric patients, or in elderly patients has not been established.
Drug Interactions
Simvastatin
The effect of multiple dose administration of EGRIFTA on the pharmacokinetics of simvastatin and simvastatin acid was evaluated in healthy subjects. Co-administration with simvastatin (a CYP3A substrate) resulted in 8% decrease in extent of absorption (AUCinf) and 5% increase in rate of absorption (Cmax) of simvastatin. For simvastatin acid there was a 15% decrease in AUCinf and 1% decrease in Cmax [see Drug Interactions (7.1)].
Ritonavir
The effect of multiple dose administration of EGRIFTA on the pharmacokinetics of ritonavir was evaluated in healthy subjects. Co-administration with ritonavir resulted in 9% decrease in AUCinf and 11% decrease in Cmax of ritonavir [see Drug Interactions (7.1)].
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of the anti-drug antibodies in other studies, including those of EGRIFTA WR or other growth hormone-releasing factor analog.
In the clinical trials with the EGRIFTA 1 mg/vial formulation, anti-tesamorelin IgG antibodies were detected in 50% of patients who received EGRIFTA for 26 weeks and 47% of patients who received EGRIFTA for 52 weeks. In the subset of patients with hypersensitivity reactions, anti-tesamorelin IgG antibodies were detected in 85%. Cross-reactivity to endogenous growth hormone-releasing hormone (GHRH) was observed in approximately 60% of patients who developed anti-tesamorelin antibodies. Patients with and without anti-tesamorelin IgG antibodies had similar mean reductions in visceral adipose tissue (VAT) and IGF-1 response. In a group of patients who had antibodies to tesamorelin after 26 weeks of treatment (56%) and were re-assessed 6 months later, after stopping EGRIFTA treatment, 18% were still antibody positive.
Neutralizing antibodies to tesamorelin and human GHRH (hGHRH) were detected in vitro at Week 52 in 10% and 5% of EGRIFTA-treated patients, respectively. Changes in VAT and IGF-1 levels in patients with or without in vitro neutralizing antibodies were comparable.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Life-time carcinogenicity studies in rodents have not been conducted with tesamorelin acetate. No potential mutagenicity of tesamorelin acetate was revealed in a battery of tests including induction of gene mutations in bacteria (the Ames test), gene mutations in mammalian cells grown in vitro (hamster CHOK1 cells), and chromosomal damage in intact animals (bone marrow cells in mice). There was no effect on fertility in male or female rats following administration of tesamorelin acetate at doses up to 0.6 mg/kg (approximately equal to clinical exposure) for 28 days in males or 14 days in females.
-
14 CLINICAL STUDIES
The safety and effectiveness of EGRIFTA WR (11.6 mg/vial formulation) has been established based on adequate and well controlled studies with EGRIFTA (1 mg/vial formulation), as well as a demonstration of comparable bioavailability between the 1.28 mg EGRIFTA WR dose (11.6 mg/vial formulation) and the 2 mg EGRIFTA dose (1 mg/vial formulation) [see Clinical Pharmacology (12.3)].
Two multicenter, randomized, double-blind, placebo-controlled studies were conducted in HIV-infected patients with lipodystrophy and excess abdominal fat (abdominal lipohypertrophy). Study 1 and Study 2 consisted of a 26-week Main Phase and a 26-week Extension Phase, respectively. Main inclusion criteria were age 18 to 65 years, a waist circumference ≥95 cm (37.4 inches) and a waist-to-hip ratio ≥0.94 for men and ≥94 cm (37.0 inches) and ≥0.88 for women, respectively, and fasting blood glucose (FBG) <150 mg/dL (8.33 mmol/L). Main exclusion criteria included BMI ≤ 20 kg/m2, type 1 diabetes mellitus, type 2 diabetes mellitus, previous treatment with insulin or with oral hypoglycemic or insulin-sensitizing agents, history of malignancy, and hypopituitarism. Patients were on a stable anti-retroviral regimen for at least 8 weeks prior to randomization. Patients meeting the inclusion/exclusion criteria were randomized in a 2:1 ratio to receive a 2 mg dose of EGRIFTA (1 mg/vial formulation) or placebo subcutaneously daily for 26 weeks. The primary efficacy assessment for each of these studies was the percent change from baseline to Week 26 in visceral adipose tissue (VAT), as assessed by computed tomography (CT) scan at L4-L5 vertebral level. Secondary endpoints included changes from baseline in patient-reported outcomes related to body image, triglycerides, ratio of total cholesterol to HDL cholesterol, IGF-1 levels, and safety parameters. Other endpoints included changes from baseline in waist circumference, abdominal subcutaneous tissue (SAT), trunk fat, and lean body mass. In both studies, EGRIFTA-treated patients completing the 26-week treatment period were re-randomized to blinded therapy with either daily placebo or a 2 mg dose of EGRIFTA (1 mg/vial formulation) for an additional 26-week treatment period (Extension Phase) in order to assess maintenance of VAT reduction and to gather long-term safety data. For inclusion in the Extension Phase studies, subjects must have completed the Main Phase with FBG ≤ 150 mg/dL.
Main Phase (Baseline to Week 26):
Study 1 (NCT 00123253)
This study randomized 412 HIV-infected patients with lipodystrophy and excess abdominal fat to receive either a 2 mg dose of EGRIFTA (1 mg/vial formulation) (N=273) or placebo (N=137). At baseline for the two groups combined, mean age was 48 years; 86% were male; 75% were white, 14% were Black/African American, and 8% were Hispanic; mean weight was 90 kg; mean BMI was 29 kg/m2; mean waist circumference was 104 cm; mean hip circumference was 100 cm; mean VAT was 176 cm2; mean CD4 cell count was 606 cells/mm3; 69% had undetectable viral load (<50 copies/mL); and 33.7% randomized to EGRIFTA and 36.6% randomized to placebo had impaired glucose tolerance, while 5.6% randomized to EGRIFTA and 6.7% randomized to placebo had diet-controlled diabetes mellitus. The twenty-six week completion rate in Study 1 was 80%.
Study 2 (NCT 00435136)
This study randomized 404 HIV-infected patients with lipodystrophy and excess abdominal fat to receive either a 2 mg dose of EGRIFTA (1 mg/vial formulation) (N=270) or placebo (N=126). At baseline for the two groups combined, mean age was 48 years; 84% were male; 77% were white, 12% were Black/African American, and 9% were Hispanic; mean weight was 88 kg; mean BMI was 29 kg/m2; mean waist circumference was 105 cm; mean hip circumference was 100 cm; mean VAT was 189 cm2; mean CD4 cell count was 592 cells/mm3; 83% had undetectable viral load (<50 copies/mL); and 44% randomized to EGRIFTA and 40% randomized to placebo had impaired glucose tolerance, while 9% randomized to EGRIFTA and 10% randomized to placebo had diet-controlled type 2 diabetes mellitus. The twenty-six week completion rate in Study 2 was 74%.
Results for the Main Phases of Studies 1 and 2 are presented in Tables 2 and 3.
Table 2: Changes from Baseline to Week 26 in Visceral Adipose Tissue (cm2) by Treatment Group (Intent-To-Treat Population with Last Observation Carried Forward) Baseline data are expressed as mean (SD); Change refers to least-squares mean (LSM); CI: confidence interval.
1 Results derived from the statistical model: Ln(VAT Week 26/VAT Baseline) = Ln(VAT Baseline) + treatment group
MAIN PHASE (Baseline-Week 26) Study 1 Study 2 2 mg EGRIFTA
(1 mg/vial)
(N=273)Placebo
(N=137)2 mg EGRIFTA
(1 mg/vial)
(N=270)Placebo
(N=126)Baseline (cm2) 178 (77) 171 (77) 186 (87) 195 (95) Change (cm2) -27 4 -21 -0 Mean treatment difference (95% CI) -31 (-39,-24) -21 (-29,-12) Mean change (%)1 -18 2 -14 -2 Mean treatment difference (95% CI)1 -20 (-24, -15) -12 (-16, -7) Table 3: Changes from Baseline to Week 26 in IGF-1, IGFBP-3, Weight, and Waist Circumference by Treatment Group (Intent-To-Treat Population with Last Observation Carried Forward) Baseline data are expressed as mean (SD); Change refers to least-squares mean (LSM); CI: confidence interval.
MAIN PHASE (Baseline-Week 26) Study 1 Study 2 2 mg EGRIFTA (1 mg/vial)
(N=273)Placebo
(N=137)2 mg EGRIFTA (1 mg/vial)
(N=270)Placebo
(N=126)IGF-1
(ng/mL)Baseline 161 (59) 168 (75) 146 (66) 149 (59) Change 107 -15 108 3 Mean treatment difference (95% CI) 122 (101, 141) 105 (85, 126) IGFBP-3
(mg/L)Baseline 3 (1) 3 (1) 3 (1) 3 (1) Change 0.4 -0.2 0.8 -0.0 Mean treatment difference (95% CI) 0.6 (0.5, 0.8) 0.8 (0.5, 1.0) Weight (kg) Baseline 90 (14) 90 (14) 89 (14) 87 (16) Change -0.4 0.0 0.5 0.3 Mean treatment difference (95% CI) -0.4 (-1.3, 0.5) 0.2 (-0.7, 1.3) Waist circumference (cm) Baseline 104 (10) 105 (9) 105 (9) 105 (9) Change -3 (5) -1 (4) -2 (5) -1 (5) Mean treatment difference (95% CI) -2 (-2.8, -0.9) -1 (-2.5, -0.3) At Week 26, treatment with a 2 mg dose of EGRIFTA (1 mg/vial formulation) resulted in a reduction from baseline in mean trunk fat of 1.0 kg in Study 1 and 0.8 kg in Study 2, respectively (compared with an increase of 0.4 kg in Study 1 and of 0.2 kg in Study 2, respectively, in patients receiving placebo). Treatment with EGRIFTA resulted in an increase from baseline in mean lean body mass of 1.3 kg in Study 1 and of 1.2 kg in Study 2, respectively (compared with a decrease of 0.2 kg in Study 1 and of 0.03 kg in Study 2, respectively, in patients receiving placebo).
Patient Reported Outcomes
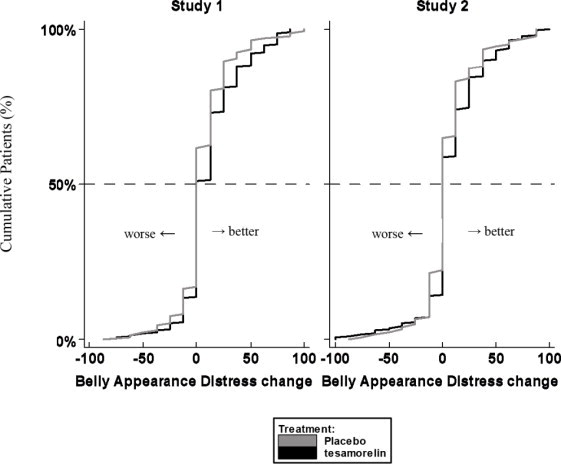
Patients rated the degree of distress associated with their belly appearance on a 9-point rating scale that was then transformed to a score from 0 (extremely upsetting and distressing) to 100 (extremely encouraging). A score of 50 indicated neutral (no feeling either way). A positive change from baseline score indicated improvement, i.e., less distress.
The cumulative distribution of response (change from baseline to 26 weeks) is shown in Figure 1 for both treatment groups. A curve shifted to the right on this scale indicates a greater percentage of patients reporting improvement.
Figure 1. Cumulative Distribution of Response for Belly Appearance Distress
Extension Phase (Weeks 26-52):
In the double-blind Extension Phase, patients on a 2 mg dose of EGRIFTA (1 mg/vial formulation) completing the 26-week Main Phase were re-randomized to receive a 2 mg dose of EGRIFTA (1 mg/vial formulation) or placebo.
Study 1 (NCT 00123253)
This study re-randomized 207 HIV-infected patients with lipodystrophy who completed a 2 mg dose of EGRIFTA (1 mg/vial formulation) treatment in the Main Phase to receive either EGRIFTA (N=154) or placebo (N=50) for an additional 26-week duration (3:1 randomization ratio). At baseline (Week 26) for the two groups combined, mean age was 48 years; 88% were male; 78% were white, 12% were Black/African American, and 8% were Hispanic; mean weight was 90 kg; mean BMI was 29 kg/m2; mean waist circumference was 102 cm; mean hip circumference was 100 cm; mean VAT was 145 cm2; mean CD4 cell count was 639 cells/mm3; 68% had undetectable viral load (<50 copies/mL); and for those EGRIFTA-treated patients completing the 26-week treatment period that were re-randomized to EGRIFTA (T-T group) or re-randomized to placebo, 37% and 32%, respectively, had impaired glucose tolerance, while 2% re-randomized to EGRIFTA and 6% re-randomized to placebo had diet-controlled type 2 diabetes mellitus. The completion rate for patients randomized into the extension phase of Study 1 was 83%.
Study 2 (NCT 00435136)
This study re-randomized 177 HIV-infected patients with lipodystrophy who completed EGRIFTA treatment in the Main Phase to receive either a 2 mg dose of EGRIFTA (1 mg/vial formulation) (N=92) or placebo (N=85) for an additional 26-week duration (1:1 randomization ratio). At baseline (Week 26) for the two groups combined, mean age was 48 years; 90% were male; 84% were white, 9% were Black/African American, and 7% were Hispanic; mean weight was 89 kg; mean BMI was 28 kg/m2; mean waist circumference was 105 cm; mean hip circumference was 100 cm; mean VAT was 172 cm2; mean CD4 cell count was 579 cells/mm3; 82% had undetectable viral load (<50 copies/mL); and for those EGRIFTA-treated patients completing the 26-week treatment period that were re-randomized to EGRIFTA (T-T group) or re-randomized to placebo, 49% and 51%, respectively, had impaired glucose tolerance, while 4% re-randomized to EGRIFTA and 13% re-randomized to placebo had diet-controlled diabetes mellitus. The completion rate for patients randomized into the extension phase of Study 2 was 81%.
Results for the Extension Phases of Studies 1 and 2 are presented in Tables 4 and 5.
Table 4: Changes from Week 26 Baseline to Week 52 in Visceral Adipose Tissue (cm2) by Treatment Group (Intent-To-Treat Population with Last Observation Carried Forward) Week 26 baseline data are expressed as mean (SD). Change refers to least-squares mean (LSM); CI: confidence interval.
1T-T = tesamorelin for Weeks 0-26 and tesamorelin for Weeks 26-52
2T-P = tesamorelin for Weeks 0-26 and placebo for Weeks 26-52
3Results derived from the statistical model: Ln(VAT Week 52/Week 26) = Ln(Week 26 VAT) + treatment group
EXTENSION PHASE (Week 26-52) Study 1 Study 2 T-T1
(Week 26-52)
(N=154)T-P2
(Week 26-52)
(N=50)T-T1
(Week 26-52)
(N=92)T-P2
(Week 26-52)
(N=85)Week 26 (cm2) 145 (72) 144 (72) 166 (89) 177 (88) Change (cm2) 3 25 -11 24 Mean treatment difference (95% CI) -22 (-34, -10) -35 (-48, -22) Mean change (%)3 0 22 -5 16 Mean treatment difference (95% CI)3 -17 (-24, -10) -18 (-24, -11) Figure 2 shows the percent change in VAT from baseline (Week 0) over time until 52 weeks in completer patients.
Figure 2. Percent Change from Baseline in VAT over Time
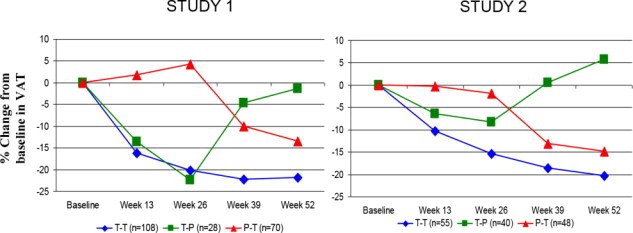
Data in Figure 2 are expressed as mean values. T-T (tesamorelin to tesamorelin) refers to the group of patients who received tesamorelin for Weeks 0-26 and were re-randomized to tesamorelin for Weeks 26-52. T-P (tesamorelin to placebo) refers to the group of patients who received tesamorelin for Weeks 0-26 and were re-randomized to placebo for Weeks 26-52. P-T (placebo to tesamorelin) refers to the group of patients who received placebo for Weeks 0-26 and were switched to tesamorelin (treated open label) for Weeks 26-52.
Table 5: Changes from Week 26 Baseline to Week 52 in IGF-1, IGFBP-3, Weight, and Waist Circumference by Treatment Group (Intent-To-Treat Population with Last Observation Carried Forward) Week 26 baseline data are expressed as mean (SD); Change refers to least-squares mean (LSM); CI: confidence interval.
1T-T = tesamorelin for Week 0-26 and tesamorelin for Week 26-52
2T-P = tesamorelin for Week 0-26 and placebo for Week 26-52
EXTENSION PHASE (Weeks 26-52) Study 1 Study 2
T-T1
(Week 26-52)
(N=154)T-P2
(Week 26-52)
(N=50)T-T1
(Week 26-52)
(N=92)T-P2
(Week 26-52)
(N=85)IGF-1
(ng/mL)Week 26 291 (124) 281 (105) 280 (134) 269 (110) Change -59 -137 -25 -135 Mean treatment difference (95% CI) 78 (50, 106) 110 (87, 134) IGFBP-3
(mg/L)Week 26 3 (1) 3 (1) 3 (1) 3 (1) Change -0.2 -0.5 -0.3 -0.9 Mean treatment difference (95% CI) 0.3 (-0.0, 0.6) 0.6 (0.3, 0.9) Weight (kg) Week 26 89 (14) 92 (17) 89 (13) 90 (14) Change 0.2 0.6 -0.5 0.1 Mean treatment difference (95% CI) -0.4 (-2, 1) -0.6 (-2, 1) Waist circumference (cm) Week 26 101 (10) 102 (12) 101 (9) 103 (11) Change -0.2 2.4 -1.1 0.2 Mean treatment difference (95% CI) -2.6 (-4, -1) -1.3 (-2, 0) Patients treated with a 2 mg dose of EGRIFTA (1 mg/formulation) for 52 weeks (T-T group) showed no change between Weeks 26 and 52 in mean trunk fat (increase of 0.1 kg in Study 1 and decrease of 0.5 kg in Study 2, respectively, compared with an increase of 1.4 kg in patients in the T-P group in Study 1 and an increase of 1.09 kg in Study 2, respectively) nor was there a change from Week 26 baseline in mean lean body mass (decrease of 0.1 kg in Study 1 and increase of 0.1 kg in Study 2, respectively, compared with a decrease of 1.8 kg in patients in the T-P group in Study 1 and a decrease of 1.7 kg in Study 2, respectively).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
EGRIFTA WR (tesamorelin) for injection is supplied as a white to off-white lyophilized powder in a 11.6 mg single-patient-use vial with a 30 mL multiple-dose bottle of Bacteriostatic Water for Injection, USP, as diluent.
EGRIFTA WR (NDC: 62064-381-04) is available in a package comprised of two boxes, containing 4 (four) 11.6 mg single-patient-use vials of EGRIFTA WR in the Medication Box and 1 (one) multiple-dose 30 mL bottles of Bacteriostatic Water for Injection, USP, diluent with a 28-day supply of disposable syringes, needles and alcohol swabs in the Injection Box.Storage and Handling
Store EGRIFTA WR 11.6 mg vial at room temperature at 20°C to 25°C (68°F to 77°F) in the original box to protect from light; excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Store the Injection box (containing Bacteriostatic Water for Injection, syringes, needles and alcohol swabs) at room temperature at 20°C to 25°C (68°F to 77°F). Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Increased Risk of Malignancy
Inform patients about the increased background risk of malignancies in HIV-positive patients and for patients with a history of neoplasms, inform them about the risk of malignancy reoccurrence [see Warnings and Precautions (5.1)].
Elevated IGF-1 Levels
Inform patients that treatment with EGRIFTA WR increases IGF-1 levels and that they will need periodic monitoring of their IGF-1 levels [see Warnings and Precautions (5.2)].
Fluid Retention
Inform patients that treatment with EGRIFTA WR may cause fluid retention, resulting in adverse reactions including edema, arthralgia, and carpal tunnel syndrome [see Warnings and Precautions (5.3)].
Glucose Intolerance or Diabetes Mellitus
Inform patients that treatment with EGRIFTA WR may result in glucose intolerance or diabetes mellitus. Advise patients that they will need to be monitored to see if impaired glucose tolerance or diabetes mellitus develops, and that if they have pre-existing diabetes mellitus, they may need adjustments to their anti-diabetic medications [see Warnings and Precautions (5.4)].
Hypersensitivity Reactions
Inform patients that hypersensitivity reactions (e.g., rash, urticaria) may occur during treatment with EGRIFTA WR. Advise patients to seek prompt medical attention and to immediately discontinue treatment with EGRIFTA WR if a reaction occurs [see Warnings and Precautions (5.5)].
Injection Site Reactions
Inform patients that injection site reactions may occur with EGRIFTA WR, including injection site erythema, pruritus, pain, irritation, and bruising. Advise patients to rotate the site of injection to reduce the risk of injection site reactions [see Warnings and Precautions (5.6)].
Pregnancy
Advise women to discontinue EGRIFTA WR if pregnancy occurs, as the drug offers no known benefit to pregnant women and could result in fetal harm [see Contraindications (4) and Use in Specific Populations (8.1)].
Lactation
Because of both the potential for HIV-1 infection transmission and serious adverse reactions in nursing infants, mothers receiving EGRIFTA WR should be instructed not to breastfeed [see Use in Specific Populations (8.2)].
Administration
Counsel patients that they should never share an EGRIFTA WR syringe with another person, even if the needle is changed. Sharing of syringes or needles between patients may pose a risk of transmission of infection.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
EGRIFTA WRTM
(tesamorelin) for injection
for subcutaneous use
11.6 mg/vialRead the Patient Information that comes with EGRIFTA WR before you start to take EGRIFTA WR and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is EGRIFTA WR?
- EGRIFTA WR is a prescription medicine used to reduce the excess stomach-area (abdominal) fat in HIV-infected adult patients with lipodystrophy. EGRIFTA WR is a growth hormone-releasing factor (GHRF).
The long-term safety of EGRIFTA WR on the heart and blood vessels (cardiovascular) is not known.
EGRIFTA WR is not for weight loss management.
It is not known whether taking EGRIFTA WR helps improve how well you take (compliance with) antiretroviral medicines.
It is not known if EGRIFTA WR is safe and effective in children.
EGRIFTA WR is not recommended to be used in children with open or closed bone growth plates (epiphyses).
Who should not use EGRIFTA WR?
Do not use EGRIFTA WR if you:
- have a pituitary gland tumor, have had pituitary gland surgery, have other problems related to your pituitary gland, or have had radiation treatment to your head or a head injury.
- have active cancer. Any previous cancer should be inactive, and any previous cancer treatment should be complete before starting EGRIFTA WR.
- are allergic to tesamorelin or any of the ingredients in EGRIFTA WR. See the end of this leaflet for a complete list of ingredients in EGRIFTA WR.
- are pregnant or plan to become pregnant. EGRIFTA WR can harm your unborn baby. If you become pregnant, stop using EGRIFTA WR and talk with your healthcare provider.
What should I tell my healthcare provider before using EGRIFTA WR?
Before using EGRIFTA WR, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had cancer.
- have problems with your blood sugar or diabetes. Some people with diabetes who use EGRIFTA WR may develop or may have worsening eye problems.
- have scheduled heart or stomach surgery.
- have breathing problems.
- are breastfeeding or plan to breastfeed. It is not known if EGRIFTA WR passes into your breast milk. The Centers for Disease Control and Prevention (CDC) recommends that HIV-infected mothers not breastfeed to avoid the risk of passing HIV infection to your baby. Talk with your healthcare provider about the best way to feed your baby if you are using EGRIFTA WR.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use EGRIFTA WR?
- Read the detailed Instructions for Use that comes with EGRIFTA WR before you start using it. Your healthcare provider will show you how to inject EGRIFTA WR.
- Use EGRIFTA WR exactly as your healthcare provider tells you to use it.
- Inject EGRIFTA WR under the skin (subcutaneously) of your stomach-area (abdomen).
- Change (rotate) the injection site on your stomach-area with each dose. Do not inject EGRIFTA WR into scar tissue, bruises or your belly button. There are two EGRIFTA formulations (EGRIFTA WR and EGRIFTA SV) with different recommended dosages. EGRIFTA WR and EGRIFTA SV are not substitutable.
- Do not share your EGRIFTA WR syringe or needles with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
What are the possible side effects of EGRIFTA WR?
EGRIFTA WR may cause serious side effects, including:
- increase risk of new cancer in HIV positive patients or your cancer coming back (reactivation). Stop using EGRIFTA WR if any cancer symptoms come back.
- increased levels of your insulin-like growth factor-1 (IGF-1). Your healthcare provider will do blood tests to check your IGF-1 levels while you are taking EGRIFTA WR.
- swelling (fluid retention). EGRIFTA WR can cause swelling in some parts of your body. Call your healthcare provider if you have swelling, an increase in joint pain or pain or numbness in your hands or wrist (carpal tunnel syndrome). Joint pain and swelling of your arms, hands, legs and feet are common side effects of EGRIFTA WR, but may sometimes be serious.
- increase in blood sugar (glucose) or diabetes. Your healthcare provider will check your blood sugar before you start taking EGRIFTA WR and during your treatment with EGRIFTA WR.
- serious allergic reaction. Some people using EGRIFTA WR may have an allergic reaction. Stop using EGRIFTA WR and get emergency medical help right away if you have any of the following symptoms:
○ a rash over your body
○ shortness of breath or trouble breathing
○ hives
○ fast heartbeat
○ itching
○ swelling of your face or throat
○ feeling of faintness or fainting
○ reddening or flushing of the skin
- injection site reactions. Injection site reactions are a common side effect of EGRIFTA WR but may sometimes be serious.Change (rotate) your injection site to help lower your risk for injection site reactions. Call your healthcare provider for medical advice if you have any of the following symptoms around the area of the injection site:
○ redness
○ irritation
○ swelling
○ itching
○ bruising or bleeding
○ pain
○ rash
- increased risk of death in people who have critical illnesses because of heart or stomach surgery, trauma or serious breathing (respiratory) problems has happened when taking certain amounts of growth hormone.
The most common side effects of EGRIFTA WR include:
pain in legs and arms
muscle pain
These are not all the possible side effects of EGRIFTA WR. For more information, ask your healthcare provider or pharmacist.Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088.
You may also report side effects to
 toll-free at 1-833-23THERA (1-833-238-4372).
toll-free at 1-833-23THERA (1-833-238-4372).How should I store EGRIFTA WR 11.6 mg vials, Bacteriostatic Water for Injection, syringes, needles and alcohol swabs?
- You will be given two boxes (Medication Box and Injection Box) from the pharmacy when you get your prescription of EGRIFTA WR:
- Store the 11.6 mg EGRIFTA WR vials in the Medication Box they come in, at room temperature between 68°F to 77°F (20°C to 25°C).
- Store the Bacteriostatic Water for Injection, syringes, needles and alcohol swabs that come in the Injection Box at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep EGRIFTA WR vials out of the light. Do not freeze.
- After mixing and injecting on the first day, keep the EGRIFTA WR vial in your Medication Box, at room temperature at 20°C to 25°C (68°F to 77°F).
- Throw away (discard) any unused EGRIFTA WR vial 7 days after mixing.
- Throw away (discard) any Bacteriostatic Water for Injection left in the bottle 28 days after first use.
- Do not use EGRIFTA WR after the expiration date (EXP) printed on the carton and vial labels.
Keep EGRIFTA WR and all medicines out of the reach of children.
General information about the safe and effective use of EGRIFTA WR.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EGRIFTA WR for a condition for which it was not prescribed. Do not give EGRIFTA WR to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about EGRIFTA WR that is written for health professionals.What are the ingredients in EGRIFTA WR?
Active ingredient: tesamorelin (as an acetate salt)
Inactive ingredients: hydrochloric acid, hydroxypropyl betadex, mannitol, sodium hydroxide
EGRIFTA WR does not contain any preservative. Bacteriostatic Water for Injection contains benzyl alcohol as preservative.Manufactured by Theratechnologies Inc., 2015 Peel Street, Suite 1100, Montréal, Québec, Canada H3A 1T8 US License No. 2091 for Theratechnologies Inc.
For more information about EGRIFTA WR, go to www.EGRIFTAWR.com or call toll-free at 1-833-23THERA (1-833-238-4372).
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 03/2025
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
EGRIFTA WRTM
(tesamorelin) for injection
for subcutaneous useNote: This Instructions for Use contains information on how to mix and inject the EGRIFTA WR 11.6 mg vial.
Read and follow the steps for weekly mixing and daily injection of EGRIFTA WR 11.6 mg vial.- Your healthcare provider should show you how to mix and inject 11.6 mg vial before you inject it for the first time. Ask your healthcare provider if you have any questions.
Important Information You Need to Know Before Injecting EGRIFTA WR 11.6 mg vial
- For subcutaneous injection only (inject directly under the skin).
- There are two EGRIFTA formulations (EGRIFTA WR and EGRIFTA SV) with different recommended dosages. EGRIFTA WR and EGRIFTA SV are not substitutable.
- The recommended daily dose of EGRIFTA WR is 1.28 mg (0.16 mL).
- One (1) EGRIFTA WR 11.6 mg vial must be mixed with 1.3 mL of the Bacteriostatic Water for Injection to prepare for 7 doses [for 7 consecutive daily injections (Day 1 to Day 7) of treatment].
- Do not share your syringe or needles with other people. You may give other people a serious infection or get a serious infection from them.
- Do not use a syringe or needle more than 1 time.
- If supplies are missing, damaged, or expired; or if you have any questions at any time during the mixing or the injection of EGRIFTA WR, call your pharmacist or toll-free at 1-833-23THERA (1-833-238-4372).

Storing EGRIFTA WR
- Store the Medication Box at room temperature between 68°F to 77°F (20°C to 25°C) and keep the EGRIFTA WR vials out of the light.
- Store the Injection Box at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze or refrigerate EGRIFTA WR after it has been mixed with the Bacteriostatic Water for Injection.
- Keep EGRIFTA WR and all medicines out of the reach of children.
Box Content
Medication Box
Supplies for 28 days:
- 4 single-patient use EGRIFTA WR 11.6 mg vials (mix only 1 vial per week as per weekly mixing)
Injection Box

Supplies for 28 days:- 1 multiple-dose, 30 mL bottle of Bacteriostatic Water for Injection (to be used for weekly mixing)
- 4 sterile BD PlastipakTM 3 mL Syringes with needle attached (clear cap), for weekly mixing
- 33 sterile BD SafetyGlideTM syringes with needle attached (orange cap), for daily injection
- 1 box of 100 alcohol swabs
Weekly Mixing (Day 1)
Getting Started
For Weekly Mixing of EGRIFTA WR, follow steps 1 through 23.
For Daily Injection (Day 1 to Day 7), follow steps 24 through 49.
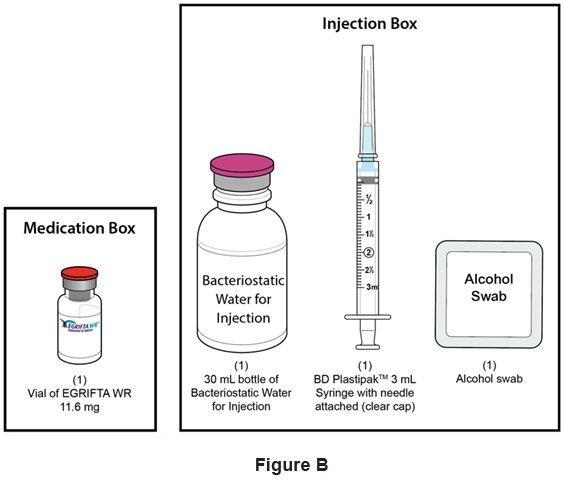
Step 1: Use a well-lit, clean, and flat surface as a working area.
Step 2: Take out the following: (See Figure B)- One (1) vial of EGRIFTA WR 11.6 mg
- One (1) 30 mL bottle of Bacteriostatic Water for Injection
- One (1) BD PlastipakTM 3 mL Syringe with needle attached (clear cap) for Weekly Mixing
- One (1) alcohol swab
- Sharps disposal container
Step 3: Wash and dry your hands well. (See Figure C)
Step 4: Inspect the compact powder in the EGRIFTA WR vial. It should be white to off-white. (See Figure D)
-
Do not use the vial if the powder is discolored or has particles in it. Get a new vial, and call your healthcare provider, pharmacist, or contact toll free at 1-833-23THERA (1-833-238-4372).

Step 5: Check the expiration dates (EXP) on the: (See Figure E)
1) EGRIFTA WR vial label
2) Bacteriostatic Water for Injection bottle
- Do not use if expired.
Step 6: Remove the plastic caps from the vial and the bottle. (See Figure F)
Step 7: Clean the top of both the vial and the bottle with an alcohol swab (See Figure G). Throw away (discard) the alcohol swab after use.
Preparing to Mix EGRIFTA WR
Step 8: Take a BD PlastipakTM 3 mL Syringe with needle attached (clear cap) out of its packaging. (See Figure H)
Step 9: Pull the protective clear needle cap straight off and throw it away (discard) (See Figure I). Make sure the needle is tightly screwed to the syringe.
- Do not twist the needle cap.
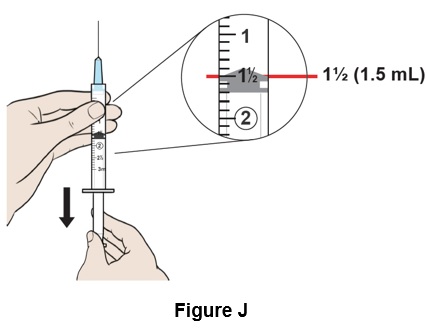
Step 10: Pull down the plunger to reach the 1½ mark (1.5 mL) on the syringe to fill it with air. (See Figure J)
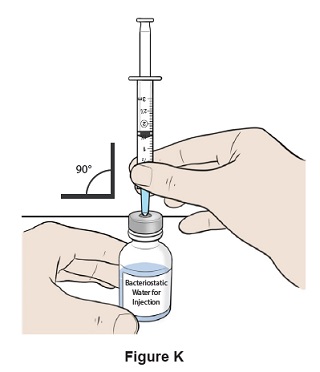
Step 11: Insert the needle straight into the top of the Bacteriostatic Water for Injection bottle. (See Figure K)
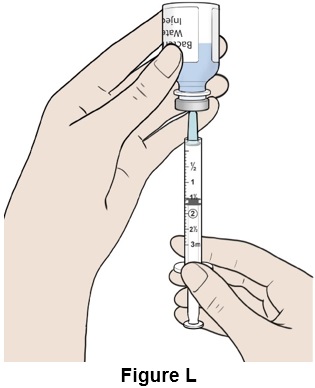
Step 12: Turn the bottle and syringe upside down. (See Figure L)
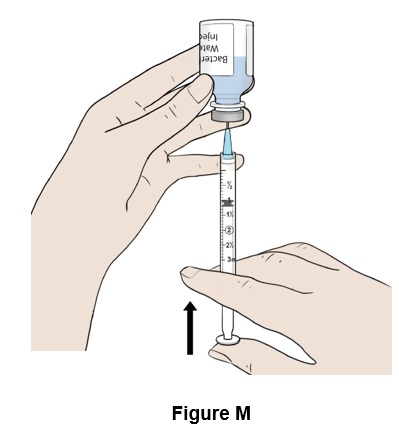
Step 13: Push the plunger all the way up (See Figure M). Some resistance may be felt.
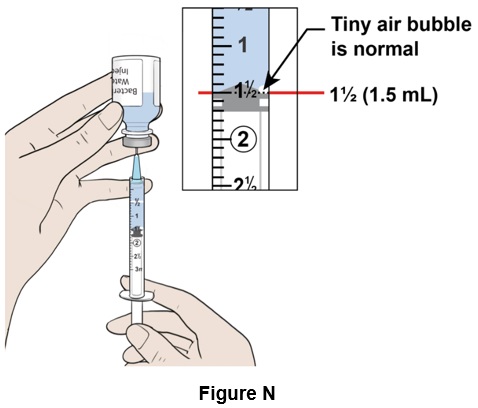
Step 14: Pull down the plunger slowly to reach the 1½ mark (1.5 mL) on the syringe. A visible air pocket and tiny air bubbles in the syringe are normal. (See Figure N)
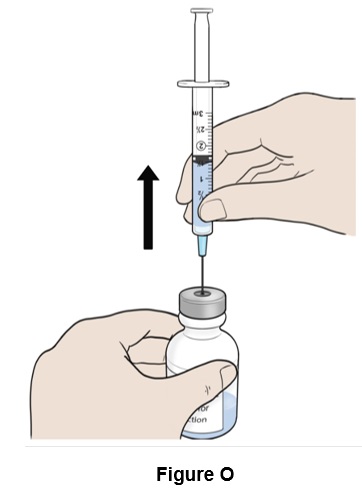
Step 15: Turn the bottle right side up and remove the needle. (See Figure O)
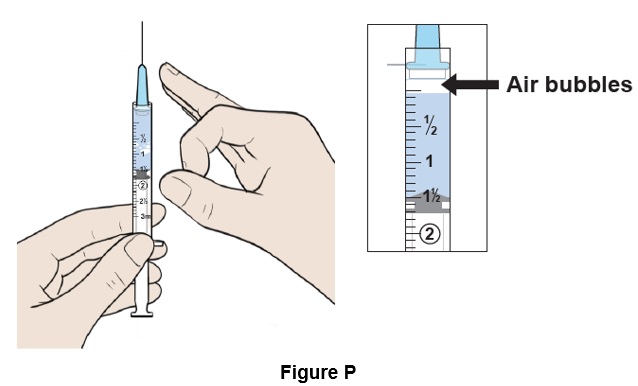
Step 16: Place the syringe upright and tap it with your finger to force any large air bubbles to rise to the top. (See Figure P)
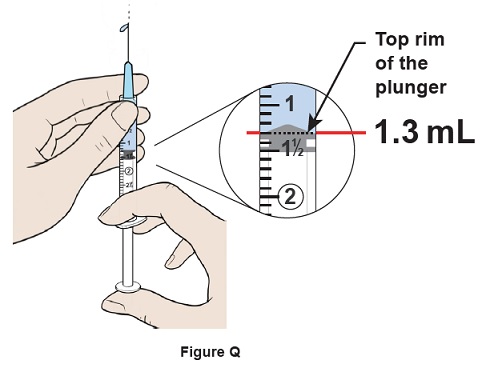
Step 17: Push the plunger up slowly to remove 0.2 mL of excess water and air out of the syringe and align the top rim of the plunger to the 1.3 mL mark. Some water should come out of the needle. (See Figure Q)
- If you have less than 1.3 mL, repeat step 10 to step 17 until 1.3 mL of water is in the syringe.
Step 18: Put the Bacteriostatic Water for Injection bottle back in the Injection box for the next Weekly Mixing. (See Figure R)
- Throw away (discard) the Bacteriostatic Water for Injection bottle after 28 days of use. Record the date of first use of the Bacteriostatic Water for Injection bottle as indicated on the back side of the Injection Box. The mixing and injection tracker provided inside the injection box can also be used to record this information.
Mixing EGRIFTA WR
Step 19: Insert the needle straight into the top of the EGRIFTA WR vial. (See Figure S)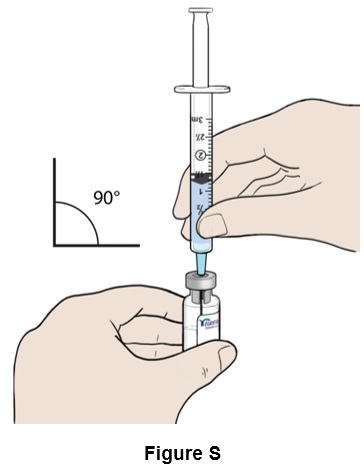
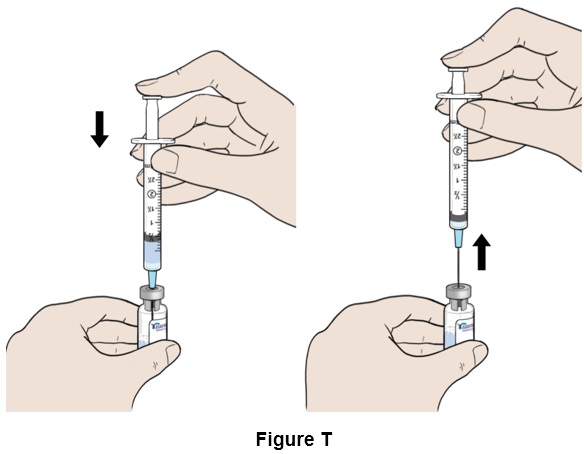
Step 20: Push the plunger down until the syringe is completely empty. While keeping the plunger down, remove the needle from the vial. (See Figure T)
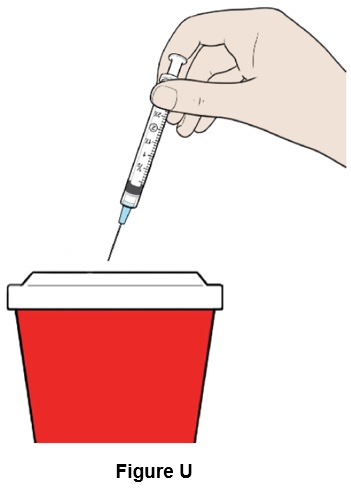
Step 21: Throw away (discard) the syringe and needle in the sharps container. (See Figure U)
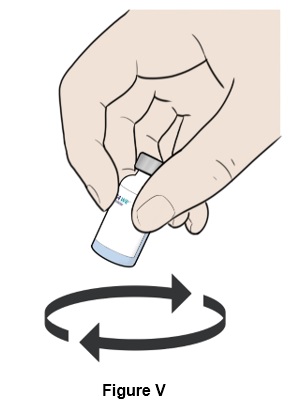
Step 22: Move the vial in a circle (swirl) to mix all the powder and liquid. (See Figure V)
- Do not shake the vial.
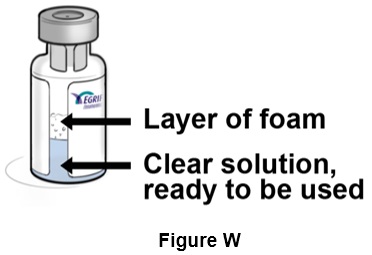
Step 23: Let the vial rest for a few seconds until the layer of foam separates and the solution becomes clear and colorless, with no particles in it. (See Figure W)
-
Do not use the vial if after a few seconds the solution looks cloudy, is discolored, or has particles in it. Get another vial, and call your healthcare provider, pharmacist, or contact toll free at 1-833-23THERA (1-833-238-4372).

Daily Injection (DAY 1 TO DAY 7)
Preparing to Inject EGRIFTA WR
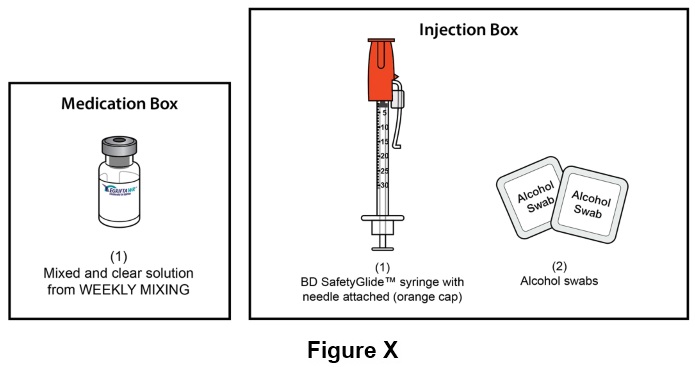
Step 24: For daily injection of EGRIFTA WR (step 24 through step 49), you will need: (See Figure X)- One (1) mixed and clear solution of EGRIFTA WR (prepared during the Weekly Mixing)
- One (1) BD SafetyGlideTM syringe with needle attached (orange cap) for injection
- Two (2) alcohol swabs
- One (1) sterile gauze pad
- One (1) adhesive bandage
- Sharps disposal container
Step 25: Wash and dry your hands well. (See Figure Y)
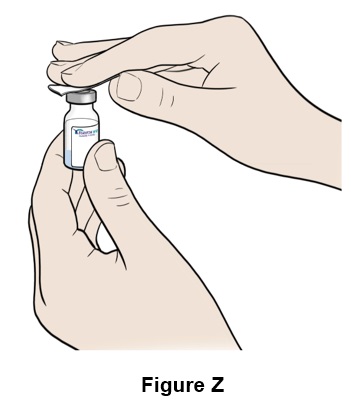
Step 26: Clean the top of the EGRIFTA WR vial with an alcohol swab (See Figure Z). Throw away (discard) the alcohol swab after use.
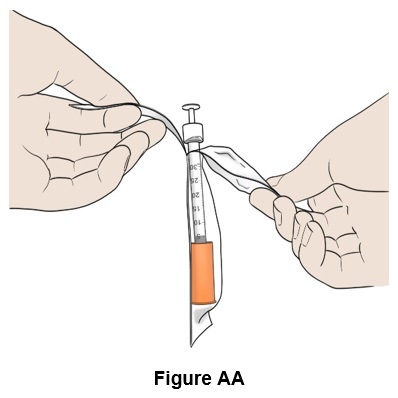
Step 27: Take a BD SafetyGlideTM syringe with needle attached (orange cap) out of its packaging. (See Figure AA)
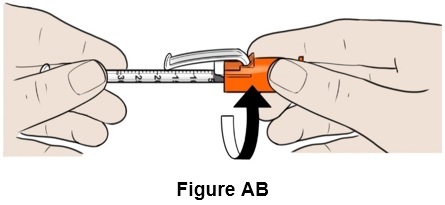
Step 28: Twist the orange needle cap to clearly see the graduated markings on the syringe, if needed. (See Figure AB)
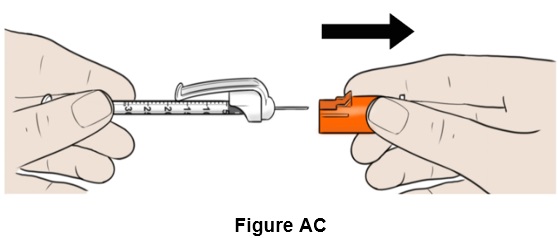
Step 29: Pull the orange needle cap straight off and keep it aside. (See Figure AC)
Do not touch the needle.
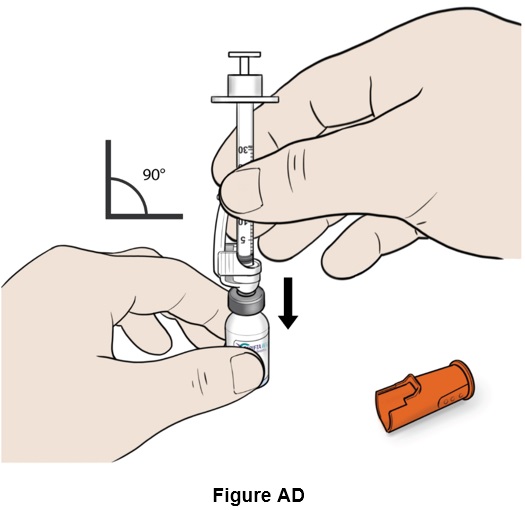
Step 30: Insert the needle carefully and straight into the top of the vial to avoid bending the needle. (See Figure AD)
- If you bend the needle, use a new syringe.
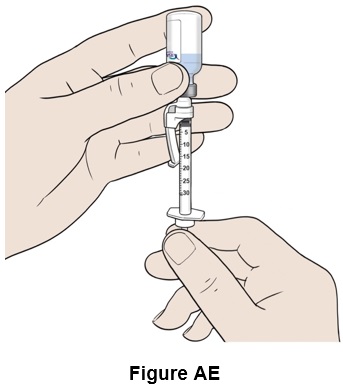
Step 31: Turn the vial and syringe upside down. (See Figure AE)
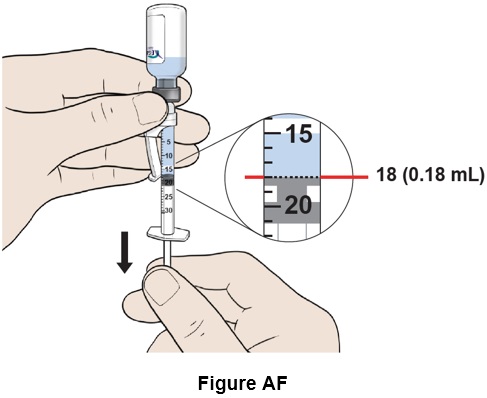
Step 32: Pull down the plunger slowly to reach the 18 mark (0.18 mL). (See Figure AF)
- If you do not have enough liquid in the syringe, repeat step 30 and step 31.
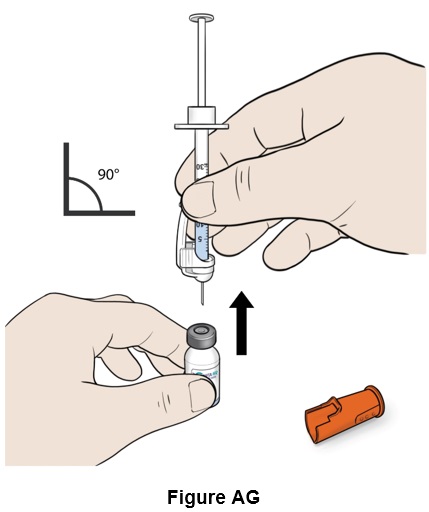
Step 33: Turn the vial right side up and remove the needle. (See Figure AG)
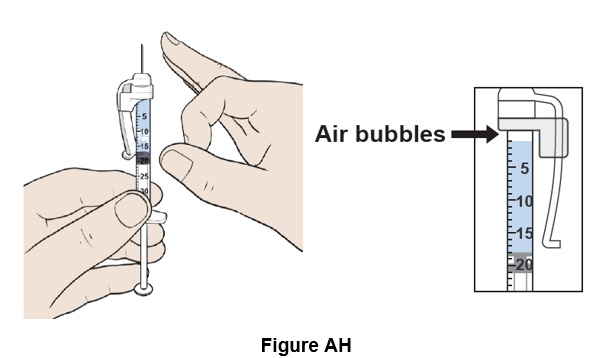
Step 34: Place the syringe upright and tap it with your finger to force any air bubbles to rise to the top. (See Figure AH)
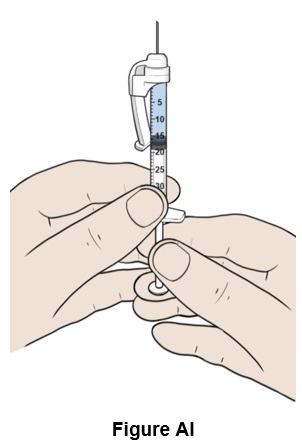
Step 35: Hold the plunger rod half-way to carefully adjust the volume in step 36. (See Figure AI)
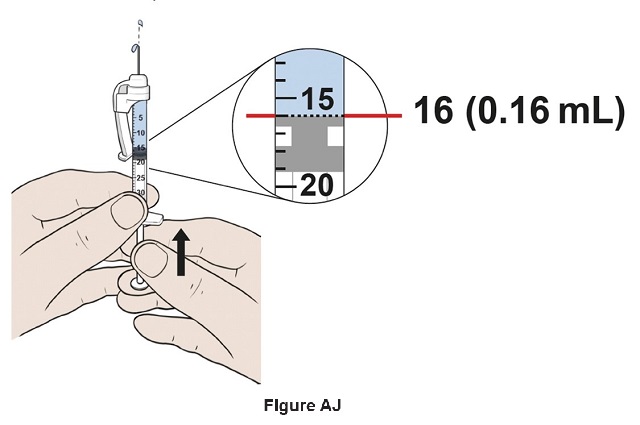
Step 36: Adjust the plunger to reach exactly the 16 mark (0.16 mL) by slowly pushing the plunger up to remove air and excess liquid from the syringe (See Figure AJ). Some resistance may be felt when pushing the plunger.
- No air pockets should be left.
Note: This is your recommended daily dose of EGRIFTA WR: 1.28 mg (0.16 mL).
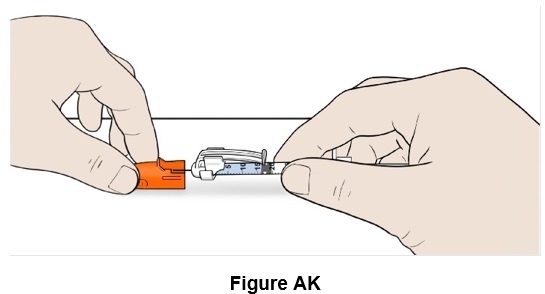
Step 37: Safely place the orange needle cap on the needle. (See Figure AK)
Step 38: Keep the mixed EGRIFTA WR vial for the next Daily Injections in the Medication Box at room temperature up to 77°F (25°C). (See Figure AL)

Injecting EGRIFTA WR
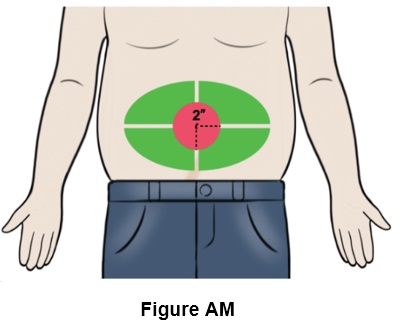
Step 39: Choose an injection site on the stomach area (abdomen) that is at least 2 inches (5 cm) away from your belly button. (See the green area in Figure AM)- Do not inject into your belly button, nor on areas with scar tissue, bruises or on any hard bumps from previous injections.
- Rotate the site for each injection. This may help prevent bruising or irritation.
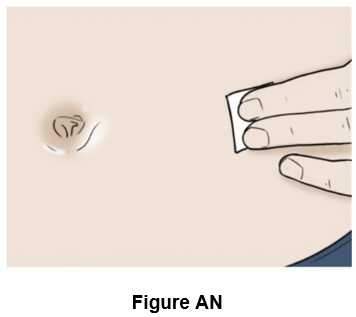
Step 40: Clean the injection site with a new alcohol swab and let it dry. (See Figure AN)
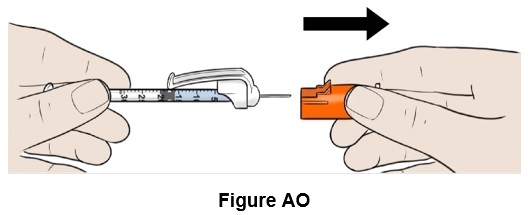
Step 41: Remove the orange needle cap and throw it away (discard). (See Figure AO)
- Do not touch the needle.
- Do not move the plunger.
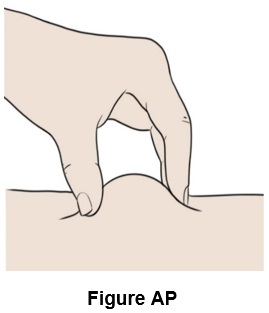
Step 42: Pinch a fold of skin at your cleaned injection site between your thumb and fingers. (See Figure AP)
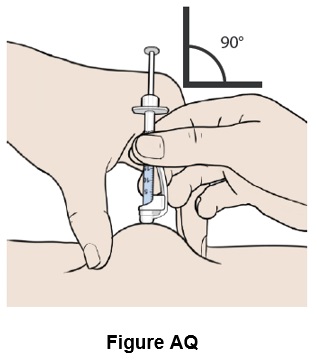
Step 43: Insert the needle straight into the skin. (See Figure AQ)
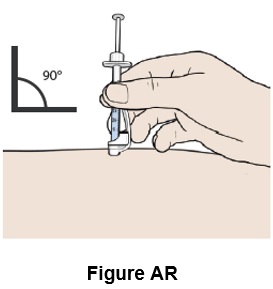
Step 44: Remove your hand from the pinched area of skin, while keeping the needle in the skin. (See Figure AR)
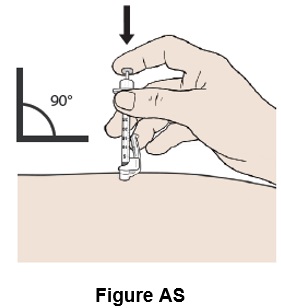
Step 45: Push the plunger all the way down until all the medicine in the syringe has been injected under the skin. (See Figure AS)
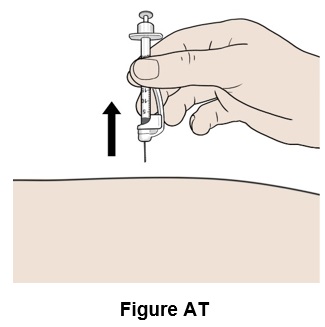
Step 46: Pull the needle out. (See Figure AT)
Step 47: Hold the syringe and carefully slide up the white safety needle shield along the needle until you hear a "click". (See Figure AU)
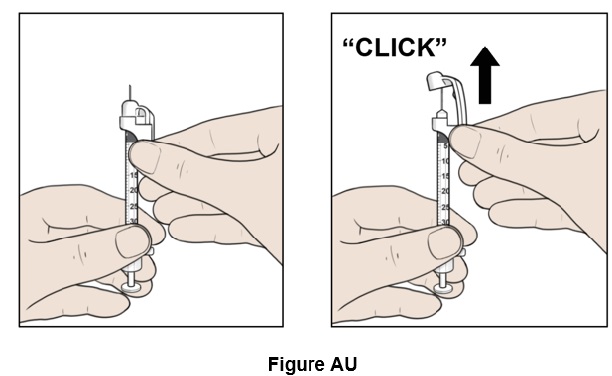
Step 48: Throw away (discard) the syringe with the needle attached into the sharps container. (See Figure AV)
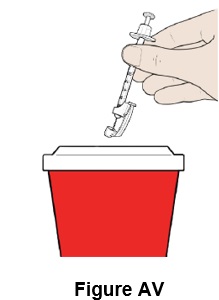
Step 49: If there is bleeding at the injection site, use gauze to apply pressure and apply an adhesive bandage if necessary.
For the next Daily Injection (Day 2 to Day 7) go directly to step 24 of this Instructions for Use.
Throw away (discard) the EGRIFTA WR vial after it has been used for 7 Daily Injections (DAY 1 TO DAY 7).
Disposing of EGRIFTA WR
- Do not recap the needle with the needle cap after you inject EGRIFTA WR.
- Throw away (discard) the EGRIFTA WR vial after it has been used for 7 Daily Injections (DAY 1 TO DAY 7).
- Throw away (discard) the Bacteriostatic Water for Injection bottle after 28 days of use.
- Put your used EGRIFTA WR needles and syringes in an FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDAs website at: https://www.fda.gov/safesharpsdisposal.
- Do not throw away (dispose of) your used sharps disposal container in your household trash unless your community guidelines permit this.
- Do not recycle your used sharps disposal container.
- If another person receives a needle stick with a used needle, that person should be told to contact a healthcare provider right away.
- Keep the sharps container away from children and pets.
-
If you have any questions, call your healthcare provider, or you can call toll free at 1-833-23THERA (1-833-238-4372) for more information.

Manufactured by: Theratechnologies Inc., 2015 Peel Street, Suite 1100, Montréal, Québec, Canada H3A 1T8
Revised: March 2025

EGRIFTA WR is a trademark of Theratechnologies Inc.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Medication Box
Rx only
MEDICATION BOX
(Box 1 of 2)
NDC: 62064-381-04
4 vials
EGRIFTA WRTM
(tesamorelin) for injection
11.6 mg/vial
For Subcutaneous Use
Single-patient-use vial
Sterile lyophilized powder
New Formulation, Dose,
Mixing Instructions, and StorageOne (1) mixed EGRIFTA WR vial provides daily doses for seven (7) days
EGRIFTA WR and EGRIFTA SV are not substitutable.
THERA
technologies
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Injection Box
INJECTION BOX
(Box 2 of 2)
For use only with EGRIFTA WR™ (tesamorelin) for injection 11.6 mg/vial
(NDC: 62064-381-04)New Formulation, Dose
Mixing Instructions, and StorageOne (1) mixed EGRIFTA WR vial
provides daily doses for seven (7) daysREAD BEFORE OPENING BOX
This INJECTION BOX does NOT contain your EGRIFTA WR
(tesamorelin) for injection 11.6 mg/vial.Make sure you also have the
EGRIFTA WR 11.6 mg/vial MEDICATION BOX.CONTENTS OF INJECTION BOX:
- 1 Instructions for Use and Patient Information
- 1 multiple-dose, 30 mL bottle of Bacteriostatic Water for Injection
- 4 sterile BD Plastipak™ 3 mL syringes with needle attached
- 33 sterile BD SafetyGlide™ syringes with needle attached
- 1 box of 100 alcohol swabs
EGRIFTA WR and EGRIFTA SV are not substitutable.
For Weekly Mixing and
Daily Injection, refer to
Instructions for Use.Store at room temperature at
20°C to 25°C (68°F to 77°F).Rx only
THERA
technologies

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 11.6 mg Vial Label
EGRIFTA WR™
(tesamorelin) for injection
11.6 mg/vial
NDC: 62064-381-01
Rx only
STERILE
Store at 20°C to 25°C
(68°F to 77°F).
For Subcutaneous Use
Single-patient-use vial
The daily recommended dose is 1.28 mg (0.16 mL).
Mfd. by Theratechnologies Inc. US License No. 2091
-
INGREDIENTS AND APPEARANCE
EGRIFTA WR
tesamorelin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62064-381 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62064-381-04 1 in 1 BOX; Type 1: Convenience Kit of Co-Package 07/15/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 4 VIAL 5.2 mL Part 2 1 BOTTLE 30 mL Part 1 of 2 EGRIFTA WR
tesamorelin injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 62064-371 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESAMORELIN ACETATE (UNII: LGW5H38VE3) (TESAMORELIN - UNII:MQG94M5EEO) TESAMORELIN 11.6 mg in 1.3 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 43.5 mg in 1.3 mL HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) 145 mg in 1.3 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Product Characteristics Color white (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62064-371-01 1.3 mL in 1 VIAL; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022505 07/15/2025 Part 2 of 2 BACTERIOSTATIC WATER
bacteriostatic water injection, solutionProduct Information Item Code (Source) NDC: 0409-3977 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 9 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018802 07/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022505 07/15/2025 Labeler - Theratechnologies Inc. (252017520)
Trademark Results [EGRIFTA WR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EGRIFTA WR 97285545 not registered Live/Pending |
Theratechnologies Inc. 2022-02-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.