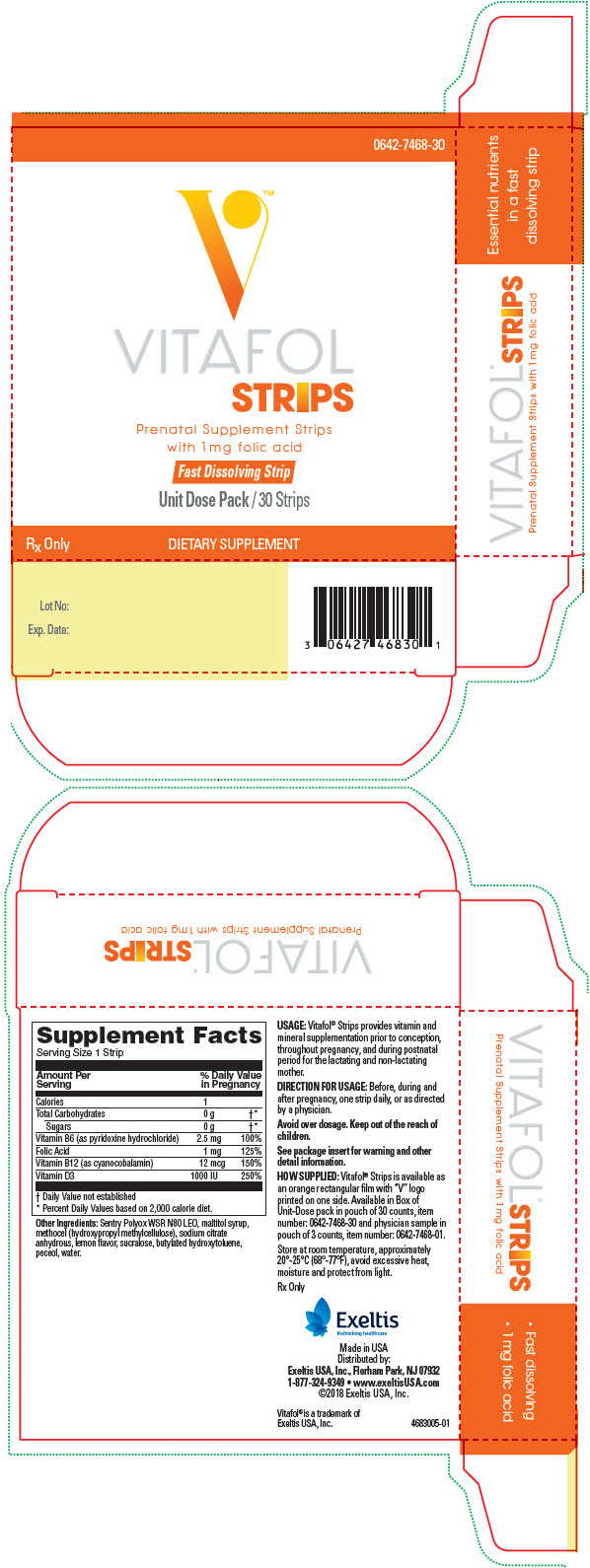
VITAFOL- cholecalciferol, pyridoxine hydrochloride, cyanocobalamin, and folic acid strip
Vitafol by
Drug Labeling and Warnings
Vitafol by is a Prescription medication manufactured, distributed, or labeled by Exeltis USA, Inc., Aquestive Therapeutics, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
COMPOSITION
Amount per daily dose (1 strip)
VITAMINS AND MINERALS: Calories 1 Total Carbohydrates 0 g Sugars 0 g Vitamin B6 (as pyridoxine hydrochloride) 2.5 mg Folic Acid 1 mg Vitamin B12 (as cyanocobalamin) 12 mcg Vitamin D (as cholecalciferol) 1000 IU Other Ingredients:
Sentry Polyox WSR N80 LEO, maltitol syrup, methocel (hydroxypropyl methylcellulose), sodium citrate anhydrous, lemon flavor, sucralose, butylated hydroxytoluene, peceol, water.
- USAGE
-
CONTRAINDICATIONS
Vitafol® Strips is contraindicated in patients with hypersensitivity to any of its components or color additives.
Folic acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (vitamin B12).
-
WARNINGS/PRECAUTIONS
This product is intended for use as directed by your healthcare provider. Please do not share with others.
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive.
The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Do not use if inner seal is broken or missing.
Do not exceed recommended dose.
Keep out of the reach of children.
DRUG INTERACTIONS
Medications for an overactive thyroid (anti-thyroid drugs) used in conjunction with iodine supplementation may lead to hypothyroidism.
Medications for hypertension used in conjunction with iodine supplementation may increase potassium.
High doses of folic acid may result in decreased serum levels of the anticonvulsant drugs; carbamazepine, fosphenytoin, phenytoin, phenobarbitol, valproic acid. Folic acid may decrease a patient's response to methotrexate.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Consult appropriate references for additional specific vitamin-drug interactions.
-
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at doses substantially higher than those in Vitafol® Strips. However, allergic and idiosyncratic reactions are possible at any dose. Reported adverse events include skin ailments, gastrointestinal complaints, glucose abnormalities, and visual problems.
- DIRECTIONS FOR USE
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 Strip Pouch Box
-
INGREDIENTS AND APPEARANCE
VITAFOL
cholecalciferol, pyridoxine hydrochloride, cyanocobalamin, and folic acid stripProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0642-7468 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLECALCIFEROL (UNII: 1C6V77QF41) (Cholecalciferol - UNII:1C6V77QF41) CHOLECALCIFEROL 1000 [iU] Pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) Pyridoxine Hydrochloride 2.5 mg Cyanocobalamin (UNII: P6YC3EG204) (Cyanocobalamin - UNII:P6YC3EG204) Cyanocobalamin 12 ug Folic Acid (UNII: 935E97BOY8) (Folic Acid - UNII:935E97BOY8) Folic Acid 1 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MALTITOL (UNII: D65DG142WK) HYPROMELLOSE 2906 (4000 MPA.S) (UNII: 5EYA69XGAT) ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) Sucralose (UNII: 96K6UQ3ZD4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) GLYCERYL OLEATE (UNII: 4PC054V79P) 1-METHYLCYCLOHEXA-1,3-DIENE (UNII: NZ9H475GT1) Product Characteristics Color ORANGE Score Shape RECTANGLE Size Flavor Imprint Code V Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0642-7468-30 30 in 1 BOX 06/15/2019 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC: 0642-7468-01 3 in 1 POUCH; Type 0: Not a Combination Product 06/15/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 06/15/2019 Labeler - Exeltis USA, Inc. (071170534) Establishment Name Address ID/FEI Business Operations Aquestive Therapeutics, Inc. 079269181 ANALYSIS(0642-7468) , MANUFACTURE(0642-7468)
Trademark Results [Vitafol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VITAFOL 88294273 not registered Live/Pending |
EXELTIS USA INC. 2019-02-08 |
 VITAFOL 86653118 4859841 Live/Registered |
Exeltis USA, Inc. 2015-06-05 |
 VITAFOL 73611865 1452861 Live/Registered |
EVERETT LABORATORIES, INC. 1986-07-28 |
 VITAFOL 72084824 0728602 Live/Registered |
VITA ZAHNFABRIK H. RAUTER, KG 1959-11-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.