RIMMEL LASTING BREATHABLE FOUNDATION SPF 20- octinoxate liquid
Rimmel Lasting Breathable Foundation SPF 20 by
Drug Labeling and Warnings
Rimmel Lasting Breathable Foundation SPF 20 by is a Otc medication manufactured, distributed, or labeled by Rimmel Inc., Coty Lancaster S.A.M.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
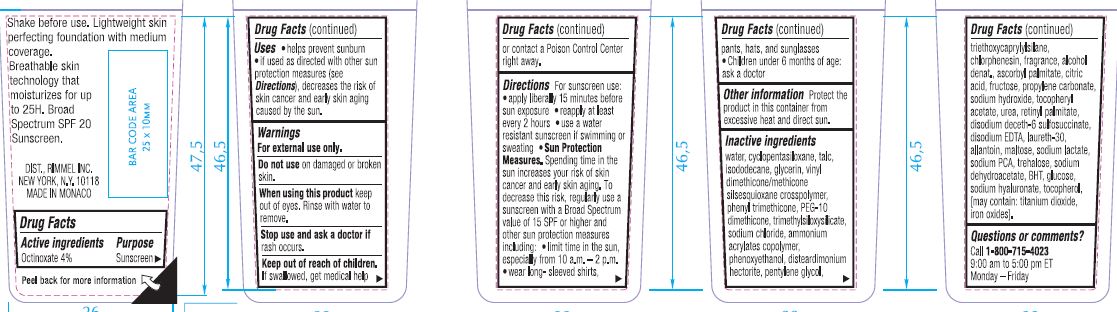
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children.
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: [bullet] limit time in the sun, especially from 10 a.m.-2 p.m. [bullet] wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age: ask a doctor
- Other information
-
Inactive ingredients
WATER, CYCLOPENTASILOXANE, TALC, ISODODECANE, GLYCERIN, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, PHENYL TRIMETHICONE, PEG-10 DIMETHICONE, TRIMETHYLSILOXYSILICATE, SODIUM CHLORIDE, AMMONIUM ACRYLATES COPOLYMER, PHENOXYETHANOL, DISTEARDIMONIUM HECTORITE, PENTYLENE GLYCOL, TRIETHOXYCAPRYLYLSILANE, CHLORPHENESIN, FRAGRANCE, ALCOHOL DENAT., ASCORBYL PALMITATE, CITRIC ACID, FRUCTOSE, PROPYLENE CARBONATE, SODIUM HYDROXIDE, TOCOPHERYL ACETATE, UREA, RETINYL PALMITATE, DISODIUM DECETH-6 SULFOSUCCINATE, DISODIUM EDTA, LAURETH-30, ALLANTOIN, MALTOSE, SODIUM LACTATE, SODIUM PCA, TREHALOSE, SODIUM DEHYDROACETATE, BHT, GLUCOSE, SODIUM HYALURONATE, TOCOPHEROL, [May Contain: TITANIUM DIOXIDE, IRON OXIDES]
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RIMMEL LASTING BREATHABLE FOUNDATION SPF 20
octinoxate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76485-1111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) GLYCERIN (UNII: PDC6A3C0OX) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TALC (UNII: 7SEV7J4R1U) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) ASCORBYL PALMITATE (UNII: QN83US2B0N) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LACTATE (UNII: TU7HW0W0QT) PROPYLENE CARBONATE (UNII: 8D08K3S51E) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PENTYLENE GLYCOL (UNII: 50C1307PZG) ALLANTOIN (UNII: 344S277G0Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76485-1111-0 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 2 NDC: 76485-1111-9 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 3 NDC: 76485-1111-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 4 NDC: 76485-1111-2 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 5 NDC: 76485-1111-4 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 6 NDC: 76485-1111-5 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 7 NDC: 76485-1111-6 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 8 NDC: 76485-1111-7 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 9 NDC: 76485-1111-8 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 10 NDC: 76485-1111-3 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/15/2019 Labeler - Rimmel Inc. (401011325) Establishment Name Address ID/FEI Business Operations Coty Lancaster S.A.M. 401011325 manufacture(76485-1111)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.