ALTREN- altrenogest solution
Altren by
Drug Labeling and Warnings
Altren by is a Animal medication manufactured, distributed, or labeled by Aurora Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE FORMS & STRENGTHS
-
VETERINARY INDICATIONS
FOR USE IN ANIMALS ONLY
For suppression of estrus in mares.
Suppression of estrus allows for a predictable occurrence of estrus following drug withdrawal in mares with ovarian follicles 20 mm or greater.
Suppression of estrus will facilitate:
Attainment of regular cyclicity during the transition from winter anestrus to the physiological breeding season.
Management of prolonged estrus conditions.
Scheduled breeding during the physiological breeding season.
FOR ORAL USE IN HORSES ONLY
- WARNING:
- CAUTION:
-
DESCRIPTION:
Altren® (altrenogest) Solution 0.22% contains the active synthetic progestin, altrenogest. The chemical name is 17α-allyl-17β-hydroxyestra-4,9,11-trien-3-one. The CAS Registry Number is 850-52-2. The chemical structure is:
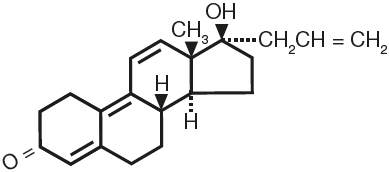
Each mL of Altren® (altrenogest) Solution 0.22% contains 2.2 mg of altrenogest in an oil solution.
- ACTIONS:
-
INDICATIONS:
Altren® (altrenogest) Solution 0.22% is indicated to suppress estrus in mares. Suppression of estrus allows for a predictable occurrence of estrus following drug withdrawal. This facilitates the attainment of regular cyclicity during the transition from winter anestrus to the physiological breeding season. Suppression of estrus will also facilitate management of prolonged estrus conditions. Suppression of estrus may be used to facilitate scheduled breeding during the physiological breeding season.
-
CONTRAINDICATIONS:
Altren® (altrenogest) Solution 0.22% is contraindicated for use in mares having a previous or current history of uterine inflammation (i.e., acute, subacute, or chronic endometritis). Natural or synthetic gestagen therapy may exacerbate existing low-grade or “smoldering” uterine inflammation into a fulminating uterine infection in some instances.
- PRECAUTIONS:
-
DOSAGE AND DIRECTIONS:
While wearing protective gloves, remove shipping cap and seal; replace with enclosed plastic dispensing cap. Remove cover from bottle dispensing tip and connect luer lock syringe (without needle). Draw out appropriate volume of Altren® solution. (Note: Do not remove syringe while bottle is inverted as spillage may result.) Detach syringe and administer solution orally at the rate of 1 mL per 110 pounds of body weight (0.044 mg/kg) once daily for 15 consecutive days. Administer solution directly on the base of the mare’s tongue or on the mare’s usual grain ration. Replace cover on bottle dispensing tip to prevent leakage. Excessive use of a syringe may cause the syringe to stick; therefore, replace syringe as necessary.
- DOSAGE CHART:
-
WHICH MARES WILL RESPOND TO ALTREN® (altrenogest) SOLUTION 0.22%:
Extensive clinical trials have demonstrated that estrus will be suppressed in approximately 95% of the mares within three days; however, the post-treatment response depended on the level of ovarian activity when treatment was initiated. Estrus in mares exhibiting regular estrus cycles during the breeding season will be suppressed during treatment; these mares return to estrus four to five days following treatment and continue to cycle normally. Mares in winter anestrus with small follicles continued in anestrus and failed to exhibit normal estrus following withdrawal.
Response in mares in the transition phase between winter anestrus and the summer breeding season depended on the degree of follicular activity. Mares with inactive ovaries and small follicles failed to respond with normal cycles post-treatment, whereas a higher proportion of mares with ovarian follicles 20 mm or greater in diameter exhibited normal estrus cycles post-treatment. Altren® (altrenogest) Solution 0.22% was very effective for suppressing the prolonged estrus behavior frequently observed in mares during the transition period (February, March and April). In addition, a high proportion of these mares responded with regular estrus cycles post-treatment.
-
SPECIFIC USES FOR ALTREN® (altrenogest) SOLUTION 0.22%:
SUPPRESSION OF ESTRUS TO:
1. Facilitate attainment of regular cycles during the transition period from winter anestrus to the physiological breeding season. To facilitate attainment of regular cycles during the transition phase, mares should be examined to determine the degree of ovarian activity. Estrus in mares with inactive ovaries (no follicles greater than 20 mm in diameter) will be suppressed but these mares may not begin regular cycles following treatment. However, mares with active ovaries (follicles greater than 20 mm in diameter) frequently respond with regular post-treatment estrus cycles.2. Facilitate management of the mare exhibiting prolonged estrus during the transition period. Estrus will be suppressed in mares exhibiting prolonged behavioral estrus either early or late during the transition period. Again, the post-treatment response depends on the level of ovarian activity. The mares with greater ovarian activity initiate regular cycles and conceive sooner than the inactive mares. Altren® (altrenogest) Solution 0.22% may be administered early in the transition period to suppress estrus in mares with inactive ovaries to aid in the management of these mares or to mares later in the transition period with active ovaries to prepare and schedule the mare for breeding.
3. Permit scheduled breeding of mares during the physiological breeding season. To permit scheduled breeding, mares which are regularly cycling or which have active ovarian function should be given Altren® (altrenogest) Solution 0.22% daily for 15 consecutive days beginning 20 days before the date of the planned estrus. Ovulation will occur 5 to 7 days following the onset of estrus as expected for non-treated mares. Breeding should follow usual procedures for mares in estrus. Mares may be regulated and scheduled either individually or in groups.
-
ADDITIONAL INFORMATION:
A 3-year well controlled reproductive safety study was conducted in 27 pregnant mares, and compared with 24 untreated control mares. Treated mares received 2 mL altrenogest solution 0.22%/110 lb body weight (2x dosage recommended for estrus suppression) from day 20 to day 325 of gestation. This study provided the following data:
1. In filly offspring (all ages) of treated mares, clitoral size was increased.
2. Filly offspring from treated mares had shorter interval from Feb. 1 to first ovulation than fillies from their untreated mare counterparts.
3. There were no significant differences in reproductive performance between treated and untreated animals (mares & their respective offspring) measuring the following parameters:
interval from Feb. 1 to first ovulation, in mares only.
mean interovulatory interval from first to second cycle and second to third cycle, mares only.
follicle size, mares only.
at 50 days gestation, pregnancy rate in treated mares was 81.8% (9/11) and untreated mares was 100% (4/4).
after 3 cycles, 11/12 treated mares were pregnant (91.7%) and 4/4 untreated mares were pregnant (100%).
colt offspring of treated and control mares reached puberty at approximately the same age (82 & 84 weeks respectively).
stallion offspring from treated and control mares showed no differences in seminal volume, spermatozoal concentration, spermatozoal motility, and total sperm per ejaculate.
stallion offspring from treated and control mares showed no difference in sexual behavior.
testicular characteristics (scrotal width, testis weight, parenchymal weight, epididymal weight and height, testicular height, width & length) were the same between stallion offspring of treated and control mares.
-
REFERENCES:
Shoemaker, C.F., E.L. Squires, and R.K. Shideler, 1989.
Safety of Altrenogest in Pregnant Mares and on Health and Development of Offspring. Eq. Vet. Sci. (9); No. 2: 69–72.
Squires, E.L., R.K. Shideler, and A.O. McKinnon. 1989.
Reproductive Performance of Offspring from Mares Administered Altrenogest During Gestation. Eq. Vet. Sci. (9); No. 2: 73–76.
- WARNING:
-
HUMAN WARNINGS:
Skin contact must be avoided as Altren® (altrenogest) Solution 0.22% is readily absorbed through unbroken skin. Protective gloves must be worn by all persons handling this product. Pregnant women or women who suspect they are pregnant should not handle Altren® (altrenogest) Solution 0.22%. Women of childbearing age should exercise extreme caution when handling this product. Accidental absorption could lead to a disruption of the menstrual cycle or prolongation of pregnancy. Direct contact with the skin should therefore be avoided. Accidental spillage on the skin should be washed off immediately with soap and water.
-
INFORMATION FOR HANDLERS:
WARNING: Altren® (altrenogest) Solution 0.22% is readily absorbed by the skin. Skin contact must be avoided; protective gloves must be worn when handling this product.
Effects of Overexposure
There has been no human use of this specific product. The information contained in this section is extrapolated from data available on other products of the same pharmacological class that have been used in humans. Effects anticipated are due to the progestational activity of altrenogest.Acute effects after a single exposure are possible; however, continued daily exposure has the potential for more untoward effects such as disruption of the menstrual cycle, uterine or abdominal cramping, increased or decreased uterine bleeding, prolongation of pregnancy and headaches. The oil base may also cause complications if swallowed.
In addition, the list of people who should not handle this product (see below) is based upon the known effects of progestins used in humans on a chronic basis.
PEOPLE WHO SHOULD NOT HANDLE THIS PRODUCT:
1. Women who are or suspect they are pregnant.
2. Anyone with thrombophlebitis or thromboembolic disorders or with a history of these events.
3. Anyone with cerebral-vascular or coronary-artery disease.
4. Women with known or suspected carcinoma of the breast.
5. People with known or suspected estrogen-dependent neoplasia.
6. Women with undiagnosed vaginal bleeding.
7. People with benign or malignant tumors which developed during the use of oral contraceptives or other estrogen-containing products.
8. Anyone with liver dysfunction or disease.
Accidental Exposure
Altrenogest is readily absorbed from contact with the skin. In addition, this oil based product can penetrate porous gloves. Altrenogest should not penetrate intact rubber or impervious gloves; however, if there is leakage (i.e., pinhole, spillage, etc.), the contaminated area covered by such occlusive materials may have increased absorption. The following measures are recommended in case of accidental exposure.Skin Exposure: Wash immediately with soap and water.
Eye Exposure: Immediately flush with plenty of water for 15 minutes. Get medical attention.
If Swallowed: Do not induce vomiting. Altren® (altrenogest) Solution 0.22% contains an oil. Call a physician. Vomiting should be supervised by a physician because of possible pulmonary damage via aspiration of the oil base. If possible, bring the container and labeling to the physician.
- STORAGE AND HANDLING
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALTREN
altrenogest solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51072-085 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALTRENOGEST (UNII: 2U0X0JA2NB) (ALTRENOGEST - UNII:2U0X0JA2NB) ALTRENOGEST 2.2 mg in 1 mL Product Characteristics Color yellow (colorless to yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51072-085-00 1000 mL in 1 BOTTLE 2 NDC: 51072-085-01 150 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200620 07/26/2017 Labeler - Aurora Pharmaceutical, Inc. (832848639) Establishment Name Address ID/FEI Business Operations Aurora Pharmaceutical, Inc. 832848639 manufacture
Trademark Results [Altren]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALTREN 86779505 5311985 Live/Registered |
AURORA PHARMACEUTICAL, INC. 2015-10-06 |
 ALTREN 77732241 not registered Dead/Abandoned |
VBP, Inc 2009-05-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.



