RILEXINE- cephalexin tablet, chewable
Rilexine by
Drug Labeling and Warnings
Rilexine by is a Animal medication manufactured, distributed, or labeled by Virbac AH, Inc, GC Hanford Manufacturing Company, ACS DOBFAR SPA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
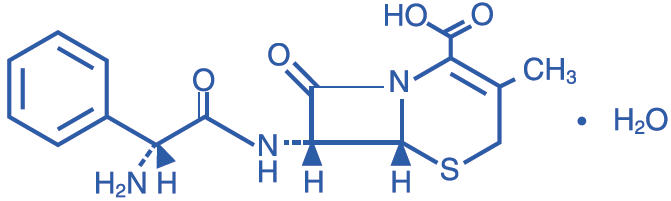
RILEXINE® Chewable Tablets are a chewable, bisected tablet supplied in 4 sizes containing 75 mg, 150 mg, 300 mg, and 600 mg of cephalexin. Cephalexin is a cephalosporin, beta-lactam, broad spectrum antibiotic. The full chemical name for cephalexin is 7-(D-α-amino- α-phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate.
- INDICATION
-
DOSAGE AND ADMINISTRATION
The recommended dose is 22 mg/kg (10 mg/lb) of body weight twice daily for 28 days.
Appropriate culture and susceptibility tests should be performed before treatment to determine the causative organism and its susceptibility to cephalexin. Therapy with RILEXINE Chewable Tablets may be initiated before results of these tests are known; once the results become available, antimicrobial therapy should be adjusted accordingly. If acceptable response to treatment is not observed, then the diagnosis should be re-evaluated and appropriate alternative therapy considered.
- CONTRAINDICATIONS
-
WARNINGS
For use in dogs only. Not for use in humans. Keep this drug out of the reach of children. Antimicrobials, including penicillins and cephalosporins, can cause allergic reactions in sensitized individuals. Sensitized individuals handling such antimicrobials, including cephalexin, should avoid contact of the product with the skin and mucous membranes in order to minimize the risk of allergic reactions.
In case of ingestion by humans contact a physician immediately. Physicians may contact a Poison Control Center for advice concerning cases of ingestion by humans.
To obtain a copy of the Material Safety Data Sheet (MSDS), or to report adverse reactions, call Virbac at 1-800-338-3659.
-
PRECAUTIONS
Prescribing antibacterial drugs in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to treated animals and may increase the risk of the development of drug-resistant animal pathogens.
The safe use of RILEXINE Chewable Tablets in dogs intended for breeding and in pregnant or lactating bitches has not been evaluated.
Positive direct Coombs' test results and false positive reactions for glucose in the urine have been reported during treatment with some cephalosporin antimicrobials. Cephalosporin antimicrobials may also cause falsely elevated urine protein determinations. Some antimicrobials, including cephalosporins, can cause lowered albumin values due to interference with certain testing methods.
Occasionally, cephalosporins have been associated with myelotoxicity, thereby creating a toxic neutropenia1. Other hematological reactions observed with cephalosporin therapy include neutropenia, anemia, hypoprothrombinemia, thrombocytopenia, prolonged prothrombin time (PT) and partial thromboplastin time (PTT), platelet dysfunction, and transient increases in serum aminotransferases2.
- 1 Birchard SJ and Sherding RG. Saunders Manual of Small Animal Practice, 2nd edition. W.B. Saunders Co. 2000: p. 166.
- 2 Adams HR. Veterinary Pharmacology and Therapeutics, 8th edition, 2001, p. 825.
-
ADVERSE REACTIONS
The most common adverse reactions in dogs include diarrhea, vomiting, anorexia and lethargy. To report suspected adverse reactions call Virbac at 1-800-338-3659.
A total of 211 dogs were included in the field study safety analysis. Adverse reactions reported in dogs treated with RILEXINE Chewable Tablets and placebo are summarized in Table 1.
Table 1: Number of Adverse Reactions* Reported During the Field Study with RILEXINE Chewable Tablets ADVERSE REACTION RILEXINE Tablets
n = 145Placebo
n = 66- * Some dogs may have experienced more than one adverse reaction or more than one occurrence of the same adverse reaction during the study.
Number of dogs with adverse reactions* 50 (34%) 22 (33%) # of Each Event* # of Each Event* Vomiting 29 9 Diarrhea 19 6 Anorexia 13 2 Lethargy 9 3 Pruritus 5 0 Dermatitis 4 3 Skin Lesions 5 1 Otitis Externa 4 2 Polydipsia 2 2 Somnolence 2 0 Flatulence 1 1 Tachypnea 1 1 No clinically significant differences were observed in the mean values for all laboratory tests including urinalysis between RILEXINE Chewable Tablets and placebo-treated dogs. At the end of treatment, group means for neutrophils, WBC, and globulin values were significantly higher in the placebo group than in the RILEXINE Chewable Tablets group; whereas, group mean values for eosinophils, A/G Ratio values, and total protein values were significantly higher in the RILEXINE Chewable Tablets group than in the placebo group. For all six of these parameters, the differences were not clinically significant and the mean values for each of the parameters remained within the normal range.
-
CLINICAL PHARMACOLOGY
Cephalexin belongs to the cephalosporin family of bactericidal antibiotics.
Cephalexin is readily and almost completely absorbed following oral administration (90% absolute bioavailability). Blood concentrations are proportional to dose within the range of at least 15 to 45 mg/kg. Binding to canine plasma proteins is low, ranging from 9 to 13% for cephalexin concentrations of 0.5 to 100 μg/mL.
Food reduces the peak cephalexin concentrations but has negligible effect on the extent of absorption.
A summary of the pharmacokinetics (PK) observed in fed and fasted Beagle dogs administered a single 22 mg/kg dose is provided in Table 2.
Table 2: Pharmacokinetics Parameter values (mean ± standard deviation), protein-corrected in fasted and fed dogs following a single administration of 22 mg/kg dose of RILEXINE Chewable Tablets (N = 12) Parameter FASTED Mean ± SD* FED Mean ± SD* - * SD = Standard Deviation
AUCINF_obs (mg.h/L) 105.36 ± 17.31 108.35 ± 25.85 AUClast (mg.h/L) 97.33 ± 13.18 95.19 ± 11.84 Cmax (mg/L) 21.66 ± 2.74 16.99 ± 2.71 T1/2(h) 7.33 ± 4.30 8.79 ± 6.44 Tmax (h) 1.42 ± 0.42 1.17 ± 0.25 Cephalosporins are associated with time-dependent killing effects. Accordingly, the pharmacodynamic (PD) target is time above MIC (T>MIC). For staphylococcal infections, the goal for time above MIC is 40% of the dosing interval (which translates to 4.8 hrs for a BID dosing schedule). For streptococcal infections, the target for time above MIC is 60% of the dosing interval (i.e., 7.2 hrs). To assess whether or not the PK-PD target is met with a 22 mg/kg BID dosing regimen under fed and fasted conditions, it was assumed that the MIC90 for S. pseudintermedius is 2 μg/mL, 8 μg/mL for S. aureus, and 0.5 μg/mL for S. canis. Plasma drug concentrations were normalized to exactly 22 mg/kg dose and corrected for 10% protein binding (protein binding observed in canine plasma).
Under fasted conditions, all targets were met in all dogs after the first daily dose. With food, the target for S. aureus was met by the second daily dose. Therefore, a 22 mg/kg BID dosing interval under fed or fasted conditions succeeded in attaining the PK-PD targets.
MICROBIOLOGY
Cephalexin is a cephalosporin antibiotic. Like other β-lactam antimicrobials, cephalexin exerts its inhibitory effect by interfering with bacterial cell wall synthesis. This interference is primarily due to its covalent binding to the penicillin-binding proteins (PBPs) (i.e., transpeptidase and carboxypeptidase), which are essential for synthesis of the bacterial wall. Minimum Inhibitory Concentrations (MICs) for cephalexin against label-claim pathogens isolated from canine pyoderma in a 2008-2009 U.S. field trial are presented in Table 3. All MICs were determined in accordance with the Clinical Laboratory Standards Institute (CLSI) standards.
Table 3: Summary of Cephalexin MIC values against S. pseudintermedius isolates from 88 dogs treated with RILEXINE® Chewable Tablets for bacterial pyoderma in a U.S. field study during 2008-2009 Microbial Treatment Outcome Time of Sampling MIC50 μg/mL MIC90 μg/mL MIC Range μg/mL - * No post-treatment sampling was conducted due to the absence of lesions.
- † Of the 27 failures, 10 did not have positive post-treatment cultures.
Success
(n = 61)*Pre-treatment 1 2 1-2 Failure
(n = 27)†Pre-treatment 1 2 1-8 Post-treatment
(n = 17)2 16 1-32 EFFECTIVENESS
The clinical effectiveness of RILEXINE Chewable Tablets was established in a randomized, multi-location, placebo-controlled field study (see Table 4). In this study, 131 dogs with secondary superficial bacterial pyoderma treated with either RILEXINE Chewable Tablets (n = 91) at 22 mg/kg (10 mg/lb) body weight or with a negative control (n = 40), twice daily for 28 days, were analyzed. RILEXINE Chewable Tablets were considered superior to the placebo (70% success rate vs. 13% respectively) in the treatment of secondary superficial bacterial pyoderma caused by susceptible strains of Staphylococcus pseudintermedius.
Table 4: Primary endpoint: Percentage of Cure* (Effectiveness population) Treatment RILEXINE Tablets Placebo p-value - * Absence of lesions at the end of the study.
N 91 40 Success 64 (70.3%) 5 (12.5%) 0.0009 Failures 27 35 Palatability
The palatability of RILEXINE Chewable Tablets was evaluated in two separate multi-location studies. In the first study, 39 client-owned dogs were dosed with RILEXINE Chewable Tablets at 22 mg/kg and evaluated for palatability of the product. Palatability testing was performed twice daily prior to feeding for 7 days. Dogs freely consumed (from empty bowl or open hand) 80.8% of their doses. In a second study, 64 client-owned dogs enrolled in the field efficacy study were evaluated in a similar manner and freely consumed 78.4% of their doses.
ANIMAL SAFETY
RILEXINE Chewable Tablets were administered orally three times a day to 12-week-old healthy Beagles at 0 mg/kg (placebo), 22 mg/kg (1×), 66 mg/kg (3×), and 110 mg/kg (5×) for 12 weeks, and at 22 mg/kg twice a day for 12 weeks. The most common clinical findings included epiphora, salivation, vomiting and diarrhea among all the dose groups. Three dogs had decreased activity (1 in each from the 22 mg/kg twice a day, 22 mg/kg three times a day, and the 66 mg/kg three times a day groups). These observations were mild and sporadic.
There were increases in alanine aminotransferase (ALT) in the 110 mg/kg three times a day group and in the 22 mg/kg twice a day group that increased in a dose-dependent pattern. There was an increase in sorbitol dehydrogenase (SDH) in the 110 mg/kg three times a day group compared to the controls. These changes were minimal and the values remained within expected historical control ranges. There were several decreases in total protein (in the 110 mg/kg three times a day group) and/or globulin (in the 22, 66, and 110 mg/kg three times a day groups) compared to the controls. These changes resulted in occasional increases in albumin/globulin ratios. Although a drug effect cannot be ruled-out, these changes were not clinically relevant.
A mild prolongation in prothrombin time (PT) was observed in the 22 mg/kg three times a day group. This was not considered clinically relevant due to the small change that remained within the reference ranges.
One dog in the 110 mg/kg three times a day group had moderate amounts of bilirubinuria at the Week 8 and Week 12 samplings. No clinical significance was noted.
Cephalexin was not present in any Day 1 samples prior to dosing or in any control animals. After dosing, cephalexin was well absorbed into systemic circulation of the treated dogs. Within gender and dosage level, Week 8 mean trough concentrations were generally higher than the Week 4 and 12 mean trough concentrations (between a 0.9 and 3.6-fold difference). The geometric mean plasma cephalexin trough concentration following three times daily administration of the 110 mg/kg dose was 11.2 μg/mL compared to 2.6 μg/mL and 8.7 μg/mL following 22 mg/kg and 66 mg/kg, respectively at Week 12. Geometric mean plasma cephalexin trough concentrations following administration of 22 mg/kg twice daily were 0.7, 1.3, and 1.0 μg/mL at Weeks 4, 8, and 12, respectively.
- STORAGE IN FORMATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 150 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 600 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
RILEXINE
cephalexin tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-026 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength cephalexin (UNII: OBN7UDS42Y) (CEPHALEXIN ANHYDROUS - UNII:5SFF1W6677) CEPHALEXIN ANHYDROUS 150 mg Product Characteristics Color WHITE (off-white to tan speckled) Score 2 pieces Shape OVAL Size 13mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-026-10 1 in 1 CARTON 1 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141326 09/01/2012 RILEXINE
cephalexin tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-036 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength cephalexin (UNII: OBN7UDS42Y) (CEPHALEXIN ANHYDROUS - UNII:5SFF1W6677) CEPHALEXIN ANHYDROUS 300 mg Product Characteristics Color WHITE (off-white to tan speckled) Score 2 pieces Shape OVAL Size 17mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-036-10 1 in 1 CARTON 1 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141326 09/01/2012 RILEXINE
cephalexin tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-046 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength cephalexin (UNII: OBN7UDS42Y) (CEPHALEXIN ANHYDROUS - UNII:5SFF1W6677) CEPHALEXIN ANHYDROUS 600 mg Product Characteristics Color WHITE (off-white to tan speckled) Score 2 pieces Shape OVAL Size 22mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-046-10 1 in 1 CARTON 1 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141326 09/01/2012 Labeler - Virbac AH, Inc (131568396)
Trademark Results [Rilexine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RILEXINE 77084766 3316330 Live/Registered |
Virbac S.A. 2007-01-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


