4213 First Aid Kit by Honeywell Safety Products USA, INC 4213 FIRST AID KIT kit
4213 First Aid Kit by
Drug Labeling and Warnings
4213 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Povidone Iodine Swab Active ingredient
- Povidone Iodine Swab Purpose
- Povidone Iodine Swab Uses
- Povidone Iodine Swab Warnings
- Povidone Iodine Swab Directions
- Povidone Iodine Swab Other information
- Povidone Iodine Swab Inactive ingredients
- Povidone Iodine Swab Questions and comments
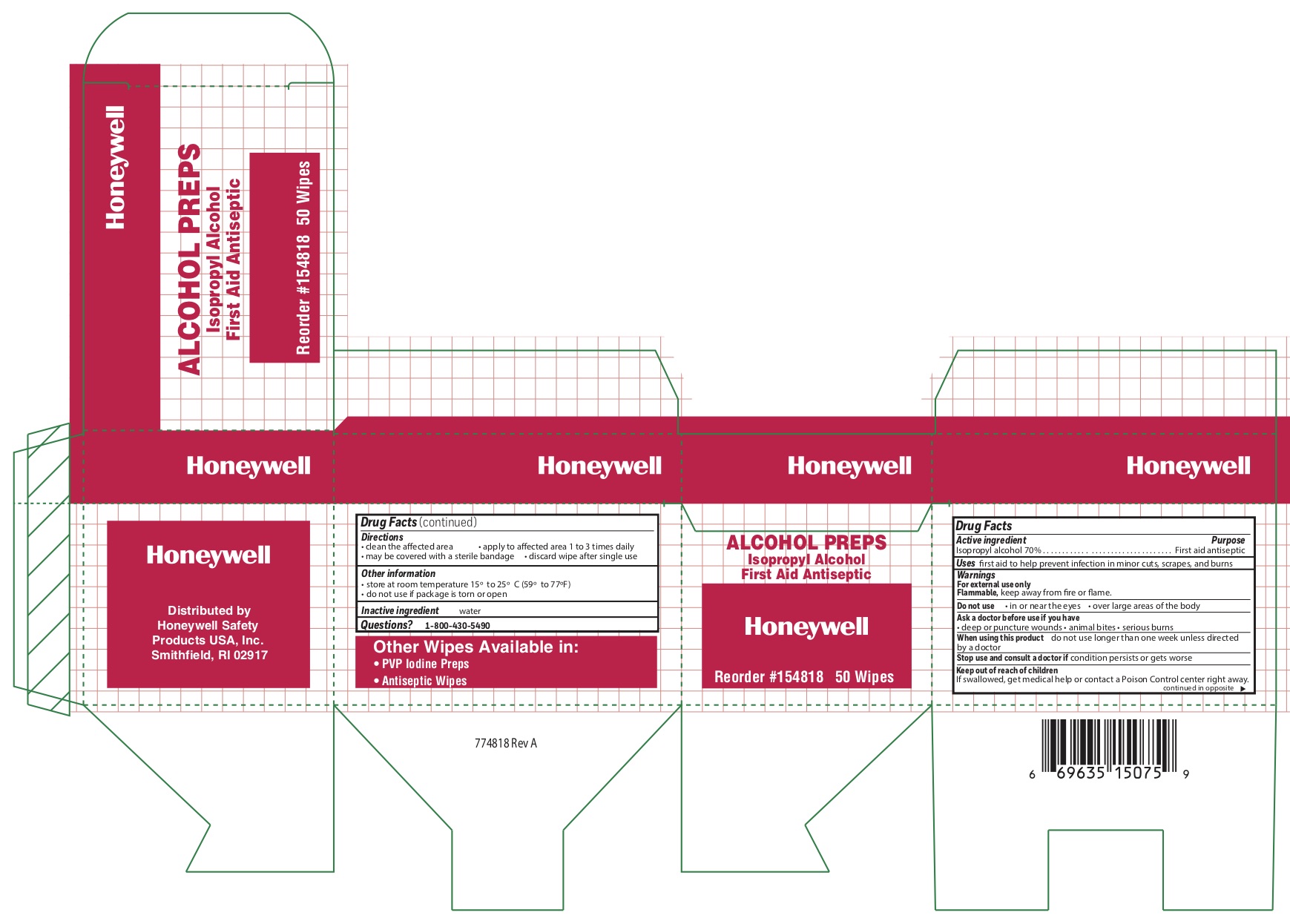
- Alcohol Wipe Active ingredient
- Alcohol Wipe Purpose
- Alcohol Wipe Uses
- Alcohol Wipe Warnings
- Alcohol Wipe Directions
- Alcohol Wipe Other information
- Alcohol Wipe Inactive ingredient
- Alcohol Wipe Questions
- Antiseptic Spray Active ingredient
- Antiseptic Spray Purpose
- Antiseptic Spray Uses
- Antiseptic Spray Warnings
- Antiseptic Spray Directions
- Antiseptic Spray Other information
- Antiseptic Spray Inactive ingredients
- Antiseptic Spray Questions
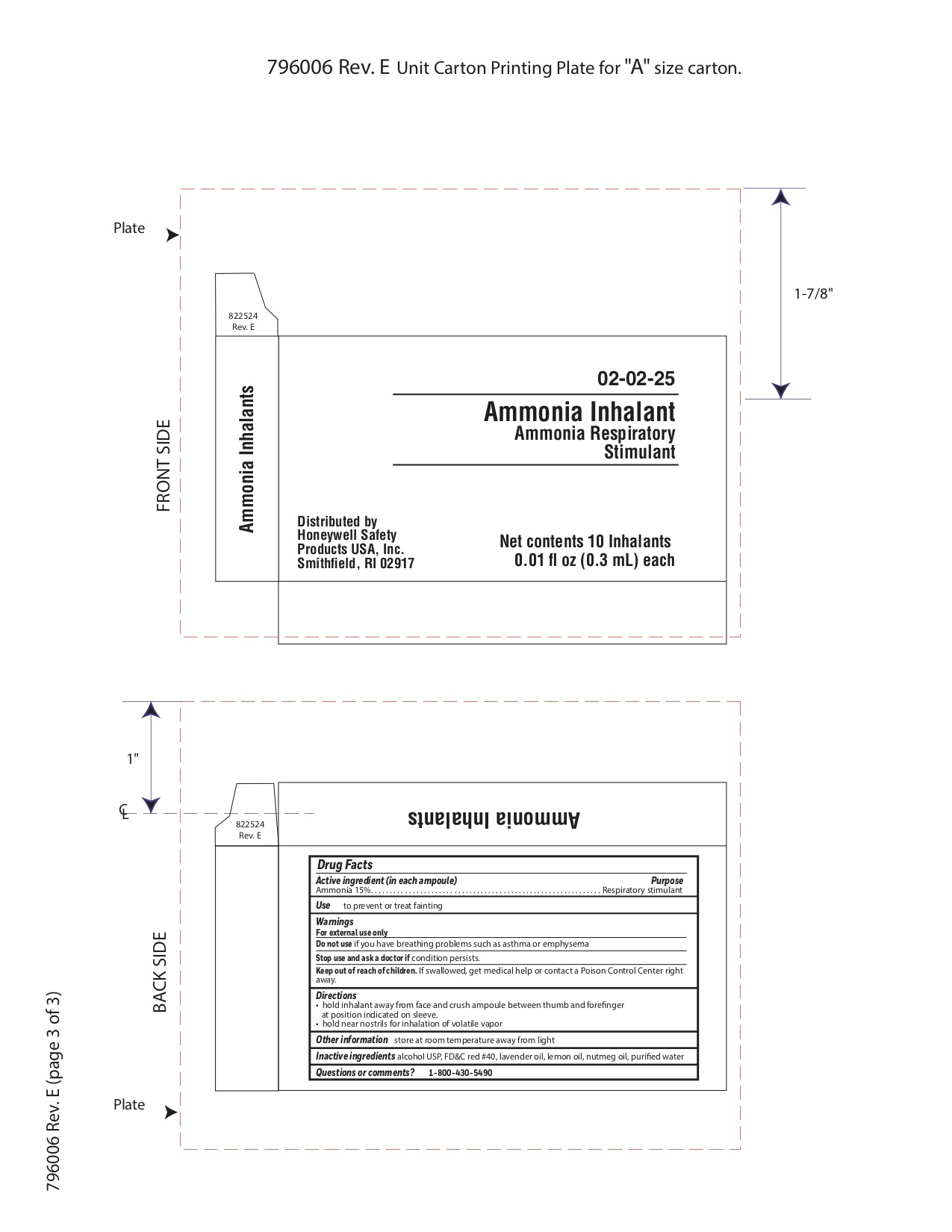
- Ammonia Inhalant Active ingredient
- Ammonia Purpose
- Ammonia Uses
- Ammonia Warnings
- Ammonia Directions
- Ammonia Other information
- Ammonia Inactive ingredient
- Ammonia Questions
- Aspirin Active Ingredient
- Aspirin Purpose
- Aspirin Uses
-
Aspirin
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for diabetes, gout or arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- any new symptoms appear
If pregnant or breast-feeding,
If pregnant or breat-feeding, ask a health professional before use. It is especially important not to use aspirin during the last three months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
- Aspirin Directions
- Aspirin Other information
- Aspirin Inactive ingredients
- Aspirin Questions or Comments?
- Povidone Iodine Swab Principal Display Panel
- Alcohol Principal Display Panel
- Antiseptic Spray Principal Display Panel
- Ammonia Principal Display Panel
- Aspirin Principal Display Panel
- 4213 Kit Label 340410F
-
4213 Kit Contnets
340410F
1 INSTANT COLD PACK 4" X 6"
1 PVP IODINE WIPES 10 PER
1 ADH TAPE W/P 1/2"X 2 1/2 YD
1 FIRST AID GUIDE ASHI
1 GAUZE CLEAN-WRAP BDGE N/S 2"
1 FIRST AID SPRAY AEROSOL 3 OZ
1 ASPIRIN 5 GR BTL OF 100'S
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 PR LRG NITRILE GLVES ZIP BAG
1 1" X 3" PLASTIC BANDS 16/BAG
4 WIPE ALCOHOL PREP IPA 70% (DUKAL)
1 KIT STL 10 UN WHITE 01
4 NON ADHERENT PAD 2" X 3"
2 AMMONIA INHALANT, BULK
-
INGREDIENTS AND APPEARANCE
4213 FIRST AID KIT
4213 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4213 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4213-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 4 POUCH 1.6 mL Part 2 10 POUCH 3 mL Part 3 1 BOTTLE, SPRAY 59 mL Part 4 2 AMPULE 0.6 mL Part 5 50 PACKET 100 Part 1 of 5 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 2 of 5 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC: 0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 3 of 5 ANTISEPTIC
benzalkonium chloride sprayProduct Information Item Code (Source) NDC: 0498-0402 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OCTOXYNOL 9 (UNII: 7JPC6Y25QS) GLYCERIN (UNII: PDC6A3C0OX) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) DIPROPYLENE GLYCOL (UNII: E107L85C40) EDETATE DISODIUM (UNII: 7FLD91C86K) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0402-59 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 09/18/2018 Part 4 of 5 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC: 0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 5 of 5 ASPIRIN
aspirin tabletProduct Information Item Code (Source) NDC: 0498-0114 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POVIDONE (UNII: FZ989GH94E) MINERAL OIL (UNII: T5L8T28FGP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code FR21 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0114-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 09/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations James Alexander 040756421 manufacture(0498-3334) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4213) Establishment Name Address ID/FEI Business Operations Ultra Seal Corporation 085752004 manufacture(0498-0114) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0402) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0143) Establishment Name Address ID/FEI Business Operations Sion Biotext Medical 532775194 manufacture(0498-0121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.