ATROPINE SULFATE- atropine sulfate injection
Atropine Sulfate by
Drug Labeling and Warnings
Atropine Sulfate by is a Prescription medication manufactured, distributed, or labeled by International Medication Systems, Limited , International Medication Systems, Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Atropine is chemically 1 α H, 5 α H - Tropan-3 α-ol (±) -tropate (ester), with the following structural formula:
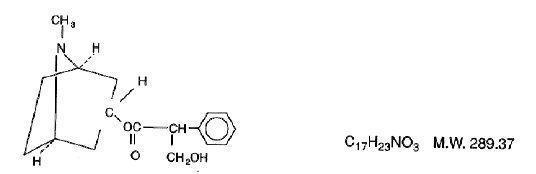
Atropine rarely occurs as such in any of the plants and has been prepared by synthesis. It is usually employed in the form of atropine sulfate (the sulfate [2:1] monohydrate salt of atropine), which has much greater solubility in water.
Atropine Sulfate Injection, USP, is a sterile aqueous solution of atropine sulfate. Each mL contains atropine sulfate, 0.1 mg; sodium chloride, 8.8 mg, for isotonicity; citric acid, 0.63 mg, and sodium citrate, 0.29 mg, as buffers. May contain additional citric acid and/or sodium citrate for pH adjustment (3.0-6.5). The air above the liquid in the container has been displaced by nitrogen gas.
-
CLlNICAL PHARMACOLOGY
Atropine inhibits the muscarinic actions of acetylcholine at postganglionic parasympathetic neuroeffector sites including smooth muscle, secretory glands and CNS sites. Large doses may block nicotinic receptors at the autonomic ganglia and at the neuromuscular junction.
Specific anticholinergic responses are dose-related. Small doses of atropine inhibit salivary and bronchial secretions and sweating; moderate doses dilate the pupil, inhibit accommodation and increase the heart rate (vagolytic effect); larger doses will decrease motility of the GI and urinary tracts; very large doses will inhibit gastric acid secretion.
-
INDICATIONS AND USAGE
Atropine Sulfate Injection, USP, may be given parenterally as a pre-anesthetic medication in surgical patients to reduce salivation and bronchial secretions. It may also be used to suppress vagal activity associated with the use of halogenated hydrocarbons during inhalation anesthesia and reflex excitation arising from mechanical stimulation during surgery.
The antispasmodic action of atropine is useful in pylorospasm and other spastic conditions of the gastrointestinal tract. For ureteral and biliary colic, atropine concomitantly with morphine may be indicated.
Atropine relaxes the upper GI tract and colon during hypotonic radiography.
In poisoning by the organic phosphate cholinesterase inhibitors found in certain insecticides and by chemical warfare nerve gases, large doses of atropine relieve the muscarine-like symptoms and some of the central nervous system manifestations. It is also used as an antidote for mushroom poisoning due to muscarine in certain species such as Amanita muscaria.
-
CONTRAINDICATIONS
Atropine Sulfate is contraindicated in patients with a history of hypersensitivity to this drug.
Ocular: Narrow-angle glaucoma; adhesions (synechiae) between the iris and lens of the eye.
Cardiovascular: Tachycardia; unstable cardiovascular status in acute hemorrhage.
GI: Obstructive disease (e.g., achalasia, pyloroduodenal stenosis, or pyloric obstruction, cardiospasm, etc.); paralytic ileus; intestinal atony of the elderly or debilitated patient; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; hepatic disease.
GU: Obstructive uropathy (e.g., bladder neck obstruction due to prostatic hypertrophy); renal disease.
Musculoskeletal: Myasthenia gravis. -
WARNINGS
Heat prostration can occur with anticholinergic drug use (fever and heat stroke due to decreased sweating) in the presence of a high environmental temperature.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. Treatment of diarrhea with these drugs is inappropriate and possibly harmful.
Usage in the elderly: Elderly patients may react with excitement, agitation, drowsiness and other untoward manifestations to even small doses of anticholinergic drugs.
Usage in gastric ulcer may produce a delay in gastric emptying time and may complicate such therapy (antral stasis).
-
PRECAUTIONS
General:
Potentially hazardous tasks: Atropine may produce drowsiness, dizziness or blurred vision; patients should observe caution while driving or performing other tasks requiring alertness.
Atropine should be used with caution in:
CNS: Autonomic neuropathy.
Ocular: Glaucoma, light irides. If there is mydriasis and photophobia, dark glasses should be worn. Atropine should be used with caution in patients over 40 years of age because of the increased incidence of glaucoma.
GI: Hepatic disease; early evidence of ileus, as in peritonitis; ulcerative colitis (large doses may suppress intestinal motility and precipitate or aggravate toxic megacolon); hiatal hernia associated with reflux esophagitis (anticholinergics may aggravate it).
GU: Renal disease; prostatic hypertrophy. Patients with prostatism can have dysuria may require catheterization.
Endocrine: Hyperthyroidism.
Cardiovascular: Coronary heart disease; congestive heart failure; cardiac arrhythmias; tachycardia; hypertension.
Usage in biliary tract disease: The use of atropine should not be relied upon in the presence of complication of biliary tract disease.
Special risk patients: Atropine should be used cautiously in infants, small children and persons with Down’s syndrome, brain damage or spasticity.
Pulmonary: Debilitated patients with chronic lung disease; reduction in bronchial secretions can lead to inspissation and formation of bronchial plugs.Atropine should be used cautiously in patients with asthma or allergies.
Drug Interactions:
Antihistamines, antipsychotics, antiparkinson drugs, alphaprodine, buclizine, meperidine, orphenadrine, benzodiazepines and tricyclic antidepressants may enhance the anticholinergic effects of atropine and its derivatives. Nitrates, nitrites, alkalinizing agents, primidone, thioxanthenes, methylphenidate, disopyramide, procainamide and quinidine may also potentiate side effects. Monoamine oxidase inhibitors block detoxification of atropine, and thus, potentiate its actions. Concurrent long-term therapy with corticosteroids or haloperidol may increase intraocular pressure. Atropine may antagonize the miotic actions of cholinesterase inhibitors.
The bronchial relaxation produced by sympathomimetics is enhanced by atropine.
Inhibition of gastric acid secretion by atropine is antagonized by guanethidine, histamine and reserpine.
Because of the potential for adverse effects, atropine should be used cautiously with digitalis, slow release digoxin tablets, cholinergics and neostigmine.
The I.V. administration of atropine in the presence of cyclopropane anesthesia can result in ventricular arrhythmias.
Atropine may enhance nitrofurantoin and thiazide-diuretic bioavailability by slowing GI motility.
The effects of metoclopramide on GI motility are antagonized by atropine.
Usage in Pregnancy:
Teratogenic Effect - Pregnancy Category B
Reproduction studies performed in mice at doses of 50 mg per kg of body weight have revealed no evidence of impaired fertility or harm to the fetus due to atropine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
GI: Xerostomia; altered taste perception; nausea; vomiting; dysphagia; heartburn, constipation; bloated feeling; paralytic ileus; gastroesophageal reflux.
GU: Urinary hesitancy and retention; impotence.
Ocular: Blurred vision; mydriasis; photophobia; cycloplegia; increased intraocular pressure.
Cardiovascular: Palpitations; bradycardia (following low doses of atropine); tachycardia (after higher doses).
CNS: Headache; flushing; nervousness; drowsiness; weakness; dizziness; insomnia; fever. Elderly patients may exhibit mental confusion or excitement to even small doses. Large doses may produce CNS stimulation (restlessness, tremor).
Dermatologic - Hypersensitivity: Severe allergic reactions including anaphylaxis, urticaria and other dermal
manifestations.
Other: Suppression of lactation; nasal congestion; decreased sweating. Complete anhidrosis cannot occur because large doses would be required, producing severe side effects from parasympathetic paralysis. -
OVERDOSAGE
Symptoms:
GI: Dry mouth; dysphagia; vomiting; nausea; abdominal distention.
CNS: Theoretically, a curare-like action may occur, i.e., neuromuscular blockade leading to muscular weakness and paralysis.
CNS stimulation; delirium; drowsiness; stupor; fever; dizziness; headache; restlessness; seizures; depression; tremor; hallucinations; ataxia; coma; psychotic behavior; other signs of an acute organic psychosis.
Cardiovascular: Circulatory failure; rapid pulse and respiration; tachycardia with weak pulse; hypertension; palpitations.
GU: Urinary urgency with difficulty in micturition.
Ocular: Blurred vision; photophobia; dilated pupils.
Miscellaneous: Leukocytosis, flushed hot dry skin, rash; respiratory failure.Treatment:
Administer supportive and symptomatic therapy as indicated.
Physostigmine 1 to 3 mg I.V. has been utilized to reverse anticholinergic effects. However, profound bradycardia, asystole and seizures may occur. The role of physostigmine is not clear; its use should be avoided if other therapeutic agents are successful in reversing cardiac dysrhythmias. Neostigmine methylsulfate 0.5 to 2 mg I.V., repeated as needed, may be given. Diazepam or short-acting barbiturates may control excitement. Hemodialysis is ineffective for atropine poisoning. Hyperpyrexia may be treated with physical cooling measures. If photophobia occurs, the patient may be kept in a dark room. -
DOSAGE AND ADMINISTRATION
Usual adult dosage:
Antimuscarinic: Intramuscular, intravenous, or subcutaneous, 400 to 600 μg (0.4 to 0.6 mg) every four to six hours.
Arrhythmias: Intravenous, 400 μg (0.4 mg) to 1 mg every one to two hours as needed, up to a maximum of 2 mg.
Gastrointestinal radiography: Intramuscular, 1 mg.
Preanesthesia (antisialagogue): Intramuscular, 200 to 600 μg (0.2 to 0.6 mg) one-half to one hour before surgery.
Cholinergic adjunct (curariform block): Intravenous, 600 μg (0.6 mg) to 1.2 mg administered a few minutes before or concurrently with 500 μg (0.5 mg) to 2 mg of neostigmine methylsulfate, using separate syringes.
Antidote (to cholinesterase inhibitors): Intravenous, 2 to 4 mg initially, then 2 mg repeated every five to ten minutes until muscarinic symptoms disappear or signs of atropine toxicity appear.
Antidote (to muscarine in mushroom poisoning): Intramuscular or intravenous, 1 to 2 mg every hour until respiratory effects subside.
Antidote (to organophosphate pesticides): Intramuscular or intravenous 1 to 2 mg, repeated in twenty to thirty minutes as soon as cyanosis has cleared. Continue dosage until definite improvement occurs and is maintained, sometimes for two days or more.Usual pediatric dosage
Antimuscarinic: Subcutaneous, 10 μg (0.01 mg) per kg of body weight, not to exceed 400 μg (0.4 mg), or 300 μg (0.3 mg) per square meter of body surface, every four to six hours.
Arrhythmias: Intravenous, 10 to 30 μg (0.01 to 0.03 mg) per kg of body weight.
Preanesthesia (antisialagogue): or
Preanesthesia (antiarrhythmic): Subcutaneous–
Children weighing up to 3 kg: 100 μg (0.1 mg).
Children weighing 7 to 9 kg: 200 μg (0.2 mg).
Children weighing 12 to 16 kg: 300 μg (0.3 mg).
Children weighing 20 to 27 kg: 400 μg (0.4 mg).
Children weighing 32 kg: 500 μg (0.5 mg).
Children weighing 41 kg; 600 μg (0.6 mg).
Antidote (to cholinesterase inhibitors): Intravenous or intramuscular, 1 mg initially, then 0.5 to 1 mg every five to ten minutes until muscarinic symptoms disappear or signs of atropine toxicity appear.NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
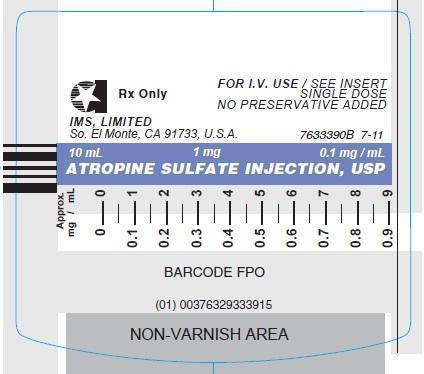
ATROPINE SULFATE INJECTION, USP.
In unit-use packages containing the Luer-Jet Luer-Lock Prefilled Syringe.
Stock No. Size NDC NO. 3339 1 mg (0.1 mg/mL) 10 mL 76329-3339-1 FOR I.V. USE Ten cartons per package.
Syringe Assembly Directions:
USE ASEPTIC TECHNIQUE
Do not assemble until ready to use.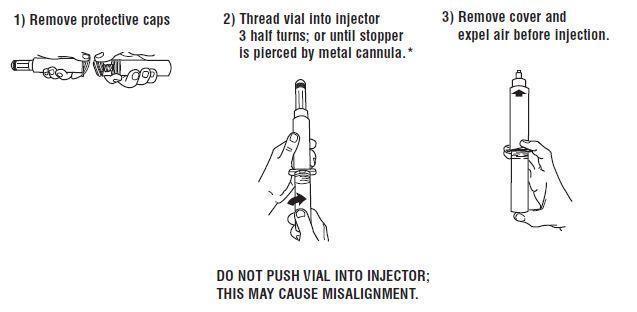
*CAUTION IMPROPER ENGAGING MAY CAUSE GLASS BREAKAGE AND SUBSEQUENT INJURY.
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx Only
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
So. El Monte, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company Rev. 2-13
© INTERNATIONAL MEDICATION SYSTEMS, LIMITED 2013 - PRINCIPLE DISPLAY PANEL: Vial Label
-
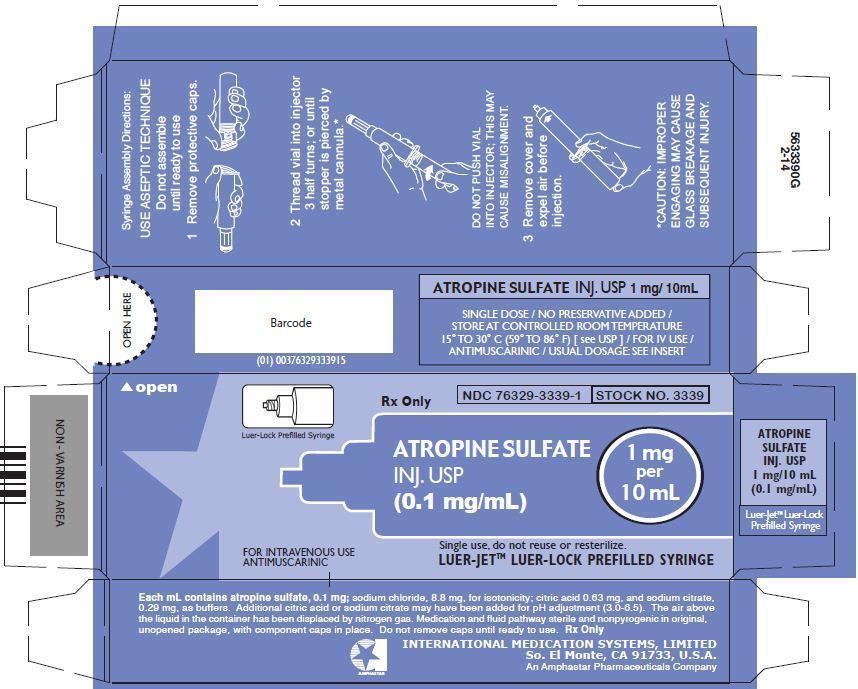
PRINCIPLE DISPLAY PANEL: Carton
Luer-Lock Prefilled Syringe
Rx Only
NDC: 76329-3339-1
STOCK NO. 3339
ATROPINE SULFATE INJ. USP (0.1 mg/mL)
1 mg per 10 mL
FOR INTRAVENOUS USE
ANTIMUSCARINIC
Single use, do not reuse or resterilize.
LUER-JETTM LUER-LOCK PREFILLED SYRINGE
-
INGREDIENTS AND APPEARANCE
ATROPINE SULFATE
atropine sulfate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 76329-3339 Route of Administration PARENTERAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Atropine Sulfate (UNII: 03J5ZE7KA5) (Atropine - UNII:7C0697DR9I) Atropine Sulfate 0.1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76329-3339-1 10 in 1 CARTON 06/01/2000 1 10 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 06/01/2000 Labeler - International Medication Systems, Limited (055750020) Establishment Name Address ID/FEI Business Operations International Medication Systems, Limited 055750020 analysis(76329-3339) , manufacture(76329-3339) , label(76329-3339)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.