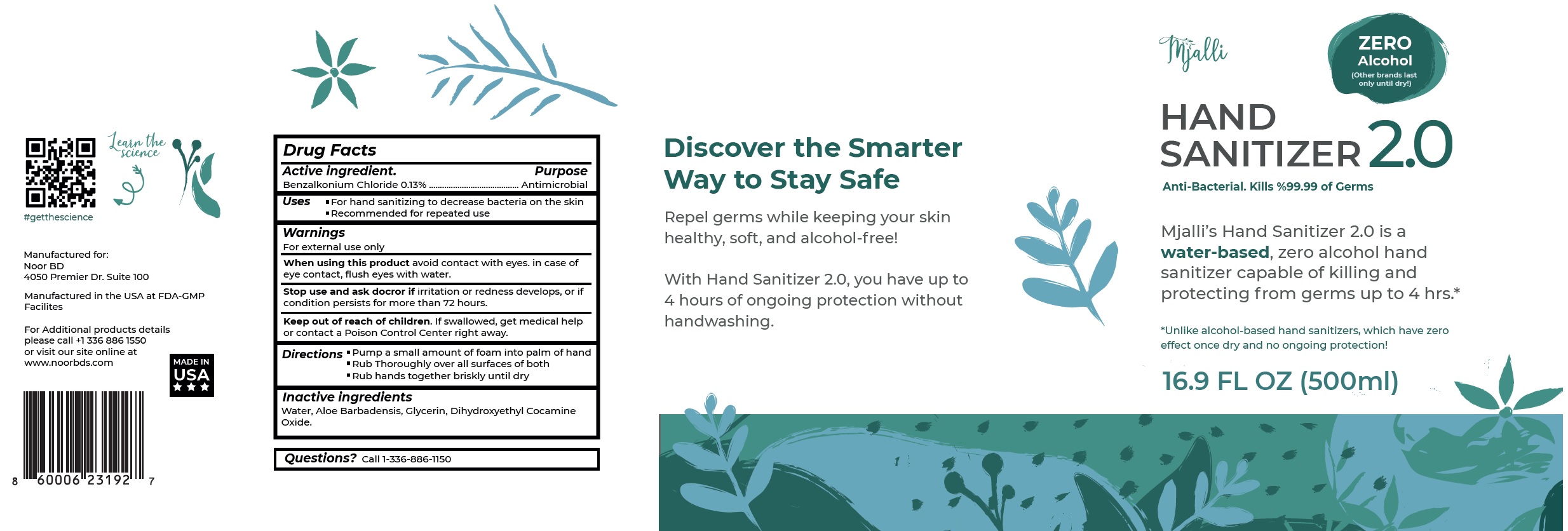
Mjalli Hand Sanitizer 2.0 by Noor Brands Company, LLC Mjalli Hand Sanitizer
Mjalli Hand Sanitizer 2.0 by
Drug Labeling and Warnings
Mjalli Hand Sanitizer 2.0 by is a Otc medication manufactured, distributed, or labeled by Noor Brands Company, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MJALLI HAND SANITIZER 2.0- benzalkonium chloride solution
Noor Brands Company, LLC
----------
Mjalli Hand Sanitizer
Warnings
For external use only
| MJALLI HAND SANITIZER 2.0
benzalkonium chloride solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Noor Brands Company, LLC (118242423) |
Revised: 1/2025
Document Id: 2cd85620-9f14-7971-e063-6394a90af1fa
Set id: 852e741c-8e28-47af-a02e-6dd8a82b6d7e
Version: 3
Effective Time: 20250129