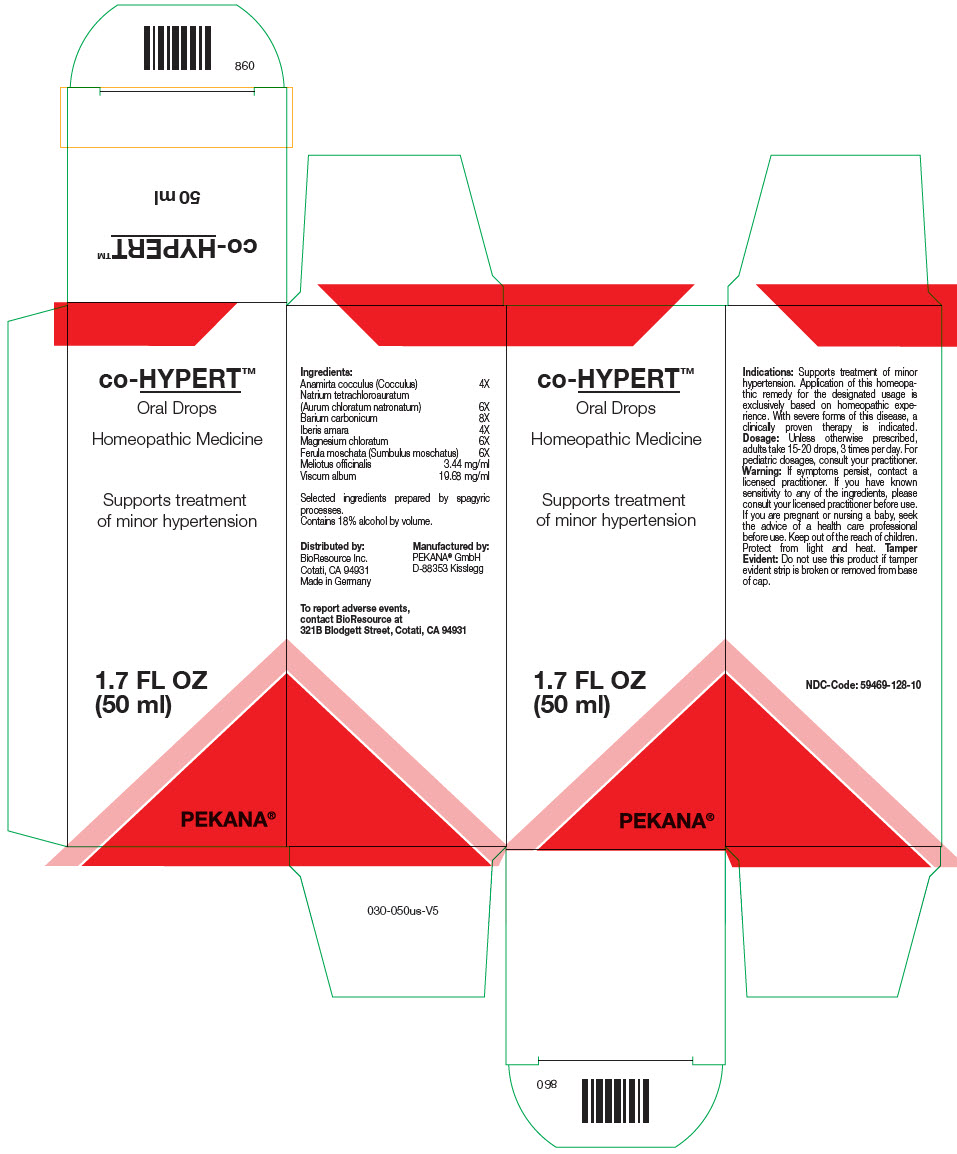
co-HYPERT by PEKANA Naturheilmittel GmbH co-HYPERT™
co-HYPERT by
Drug Labeling and Warnings
co-HYPERT by is a Homeopathic medication manufactured, distributed, or labeled by PEKANA Naturheilmittel GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CO-HYPERT- sodium tetrachloroaurate, barium carbonate, anamirta cocculus seed, iberis amara seed, magnesium chloride, ferula sumbul root, melilotus officinalis top, and viscum album fruiting top solution/ drops
PEKANA Naturheilmittel GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
co-HYPERT™
| Ingredients: | |
|---|---|
| Anamirta cocculus (Cocculus) | 4X |
| Natrium tetrachloroauratum (Aurum chloratum natronatum) | 6X |
| Barium carbonicum | 8X |
| Iberis amara | 4X |
| Magnesium chloratum | 6X |
| Ferula moschata (Sumbulus moschatus) | 6X |
| Meliotus officinalis | 3.44 mg/ml |
| Viscum album | 19.68 mg/ml |
Selected ingredients prepared by spagyric processes.
Dosage
Unless otherwise prescribed, adults take 15-20 drops, 3 times per day. For pediatric dosages, consult your practitioner.
| CO-HYPERT
sodium tetrachloroaurate, barium carbonate, anamirta cocculus seed, iberis amara seed, magnesium chloride, ferula sumbul root, melilotus officinalis top, and viscum album fruiting top solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - PEKANA Naturheilmittel GmbH (320344542) |