DOCTOR SAFE- alcohol gel
DOCTOR SAFE by
Drug Labeling and Warnings
DOCTOR SAFE by is a Otc medication manufactured, distributed, or labeled by ONE DIAMOND ELECTRONICS, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
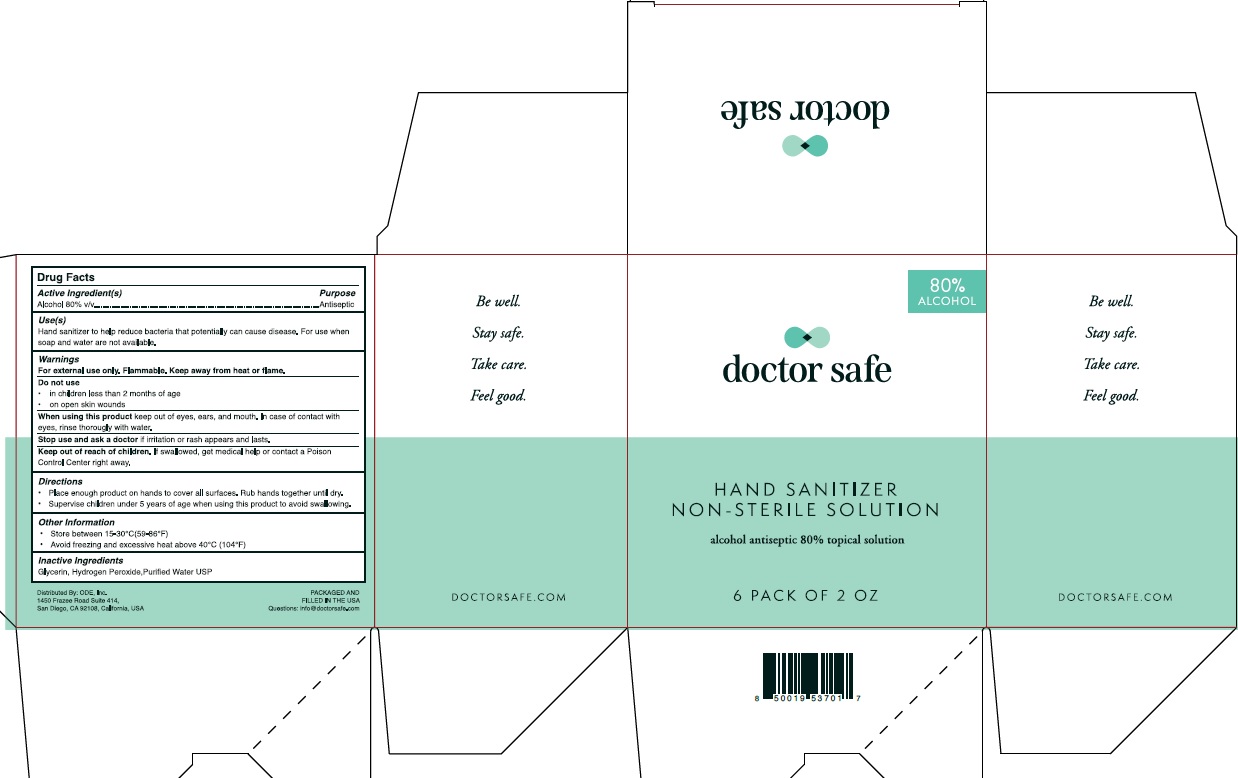
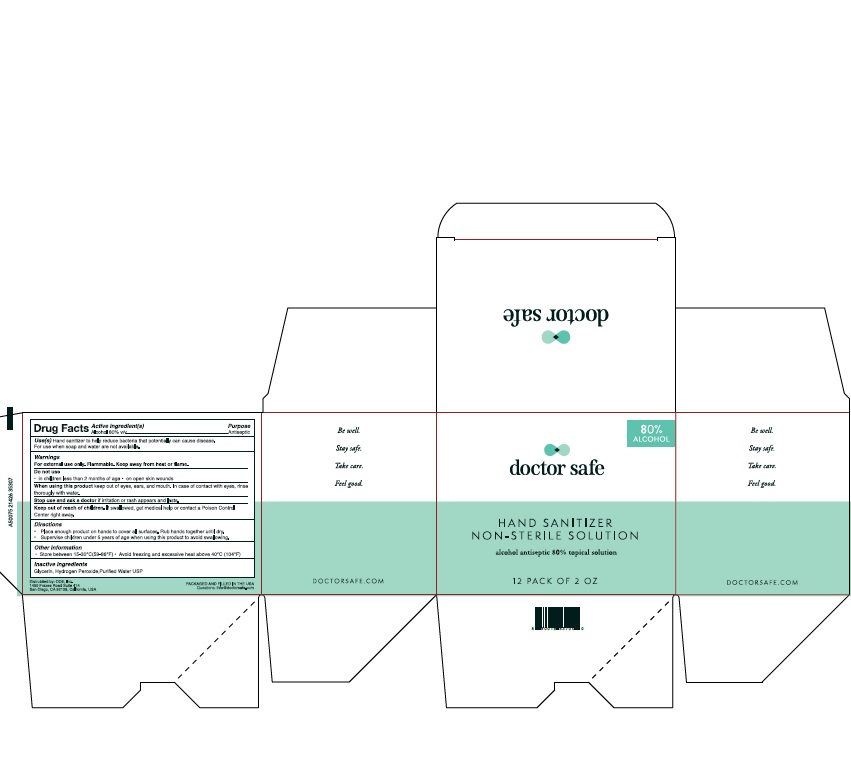
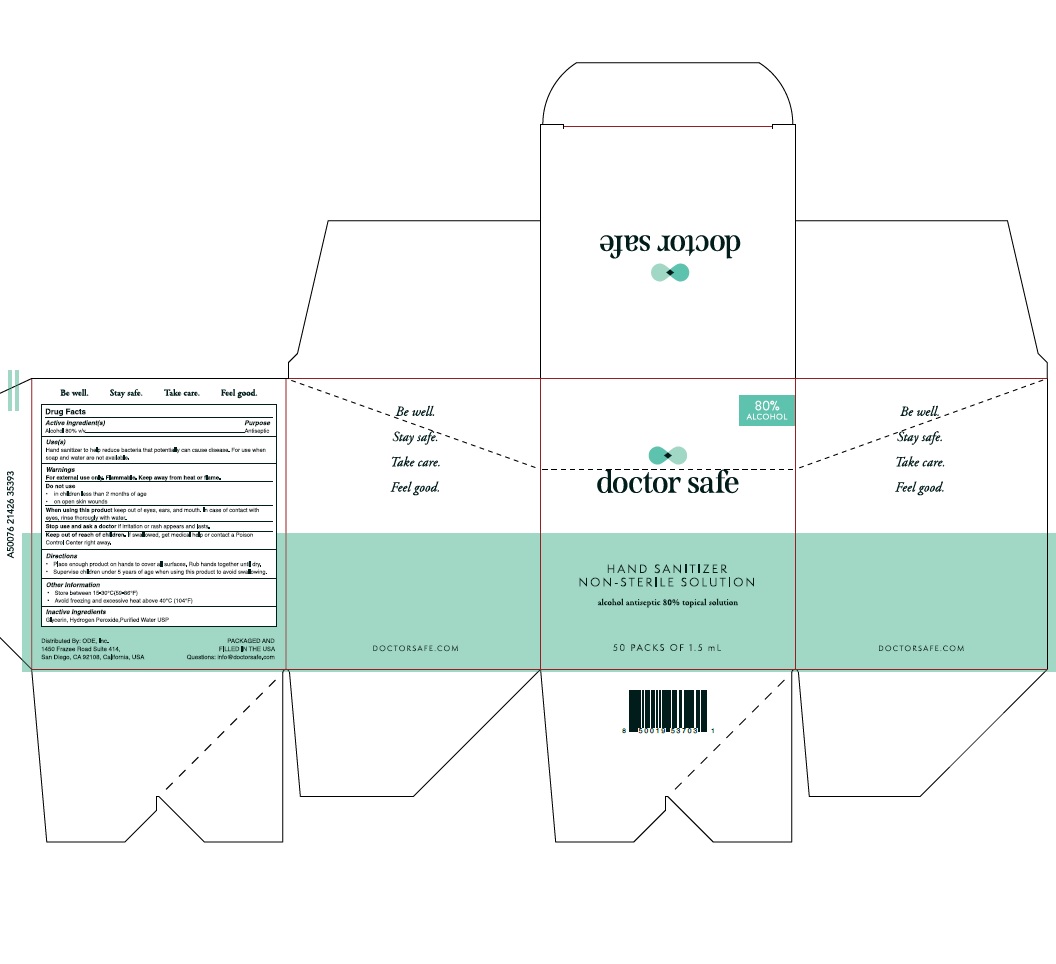
- Active Ingredient
- Purpose
-
Warnings
For external use only. Flammable. Keep away from heat or flame.
Do not use:
1) in children less than 2 months of age;
2) on open skin wounds.
When using this product keep out of eyes, ears, and mouth.
In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs.
These may be signs of a serious condition.
If swallowed, get medical help, or contact a Poison Control Center right away.
- Uses
- Directions
- Keep out of reach of children.
- Other Information
- Inactive Ingredient
- 1.5mL
- 6Packs
- 12Packs
- 50packs
- 59mL Bottle
- 236mL
-
INGREDIENTS AND APPEARANCE
DOCTOR SAFE
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 78542-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 80 mL in 100 mL Inactive Ingredients Ingredient Name Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 78542-001-01 1.5 mL in 1 POUCH; Type 0: Not a Combination Product 06/10/2020 2 NDC: 78542-001-02 6 in 1 BOX 06/01/2020 2 59 mL in 1 PACKAGE; Type 0: Not a Combination Product 3 NDC: 78542-001-03 12 in 1 BOX 06/01/2020 3 59 mL in 1 PACKAGE; Type 0: Not a Combination Product 4 NDC: 78542-001-04 50 in 1 BOX 06/01/2020 4 1.5 mL in 1 POUCH; Type 0: Not a Combination Product 5 NDC: 78542-001-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2020 6 NDC: 78542-001-06 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/01/2020 Labeler - ONE DIAMOND ELECTRONICS, INC. (086508293)
Trademark Results [DOCTOR SAFE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOCTOR SAFE 98040674 not registered Live/Pending |
Doctor Safe Holdings Inc. 2023-06-13 |
 DOCTOR SAFE 98040664 not registered Live/Pending |
Doctor Safe Holdings Inc. 2023-06-13 |
 DOCTOR SAFE 88872372 not registered Live/Pending |
One Diamond Electronics, Inc 2020-04-15 |
 DOCTOR SAFE 88862690 not registered Live/Pending |
One Diamond Electronics, Inc 2020-04-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.