nystatin by E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. / Fougera Pharmaceuticals Inc. NYSTATIN suspension
nystatin by
Drug Labeling and Warnings
nystatin by is a Prescription medication manufactured, distributed, or labeled by E. Fougera & Co. a division of Fougera Pharmaceuticals Inc., Fougera Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
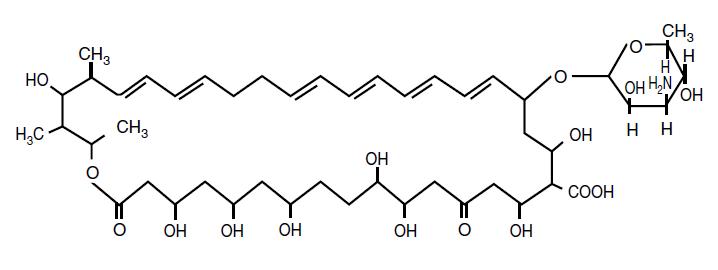
Nystatin is an antimycotic polyene antibiotic obtained from Streptomyces noursei. The structural formula is:
Molecular Formula: C47 H75 NO17 Molecular Weight: 926.13
Nystatin Oral Suspension, for oral administration, contains 100,000 USP Nystatin Units per mL. Inactive ingredients: alcohol USP (not more than 1% by volume), mint blend flavoring, dibasic sodium phosphate USP, glycerin USP, purified water USP, colloidal silicon dioxide, sucrose NF (50%), methylparaben NF (0.12%) and propylparaben NF (0.03%) as preservatives.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics:
Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving oral therapy with conventional dosage forms, significant plasma concentrations of nystatin may occasionally occur.
Microbiology:
Nystatin is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi. Candida albicans demonstrates no significant resistance to nystatin in vitro on repeated subculture in increasing levels of nystatin; other Candida species become quite resistant. Generally, resistance does not develop in vivo. Nystatin acts by binding to sterols in the cell membrane of susceptible Candida species with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General:
This medication is not to be used for the treatment of systemic mycoses. Discontinue treatment if sensitization or irritation is reported during use.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No long-term animal studies have been performed to evaluate carcinogenic potential. There also have been no studies to determine mutagenicity or whether this medication affects fertility in males or females.
Pregnancy: Teratogenic Effects–Pregnancy Category C. Animal reproduction studies have not been conducted with nystatin oral suspension. It is also not known whether nystatin oral suspension can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Nystatin oral suspension should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported.
(See PRECAUTIONS, General.)
Gastrointestinal: Diarrhea (including one case of bloody diarrhea), nausea, vomiting, gastrointestinal upset/disturbances.
Dermatologic: Rash, including urticaria has been reported rarely. Stevens-Johnson syndrome has been reported very rarely.
Other: Tachycardia, bronchospasm, facial swelling, and nonspecific myalgia have also been rarely reported.
-
OVERDOSAGE
Oral doses of nystatin in excess of five million units daily have caused nausea and gastrointestinal upset. There have been no reports of serious toxic effects or superinfections (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
-
DOSAGE AND ADMINISTRATION
INFANTS: 2 mL (approximately 1/2 teaspoon)(200,000 units) four times daily 1 mL (approximately 1/4/ teaspoon) (one-half of dose) in each side of mouth and avoid feeding for 5 to 10 minutes.
NOTE: Limited clinical studies in premature and low birth weight infants indicate that 1 mL four times daily is effective.CHILDREN AND ADULTS: 4 - 6 mL (approximately 1 teaspoon)(400,000 to 600,000 units) four times daily (one-half of dose in each side of mouth). The preparation should be retained in the mouth as long as possible before swallowing.
Continue treatment for at least 48 hours after perioral symptoms have disappeared and cultures demonstrate eradication of Candida albicans.
-
HOW SUPPLIED
Nystatin Oral Suspension, USP (100,000 USP Nystatin Units per mL) is available as a mint-flavored, light yellow, ready-to-use suspension in the following sizes:
NDC: 0168-0037-60 60 mL bottle (with a calibrated dosing cup)
NDC: 0168-0037-61 60 mL bottle (with a calibrated dropper)
NDC: 0168-0037-74 1 Pint bottleShake well before using. Wash cup before and after each use.
Before dispensing, replace cap with safety cap dropper.Store at 20°-25°C (68°-77°F); excursions permitted between 15°-30°C (59°-86°F)
[see USP Controlled Room Temperature].WARNING: Keep out of reach of children.
This product sealed for your protection. If the seal is missing or broken return to place of purchase.
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747I237C/IF237E
R11/07
#276 -
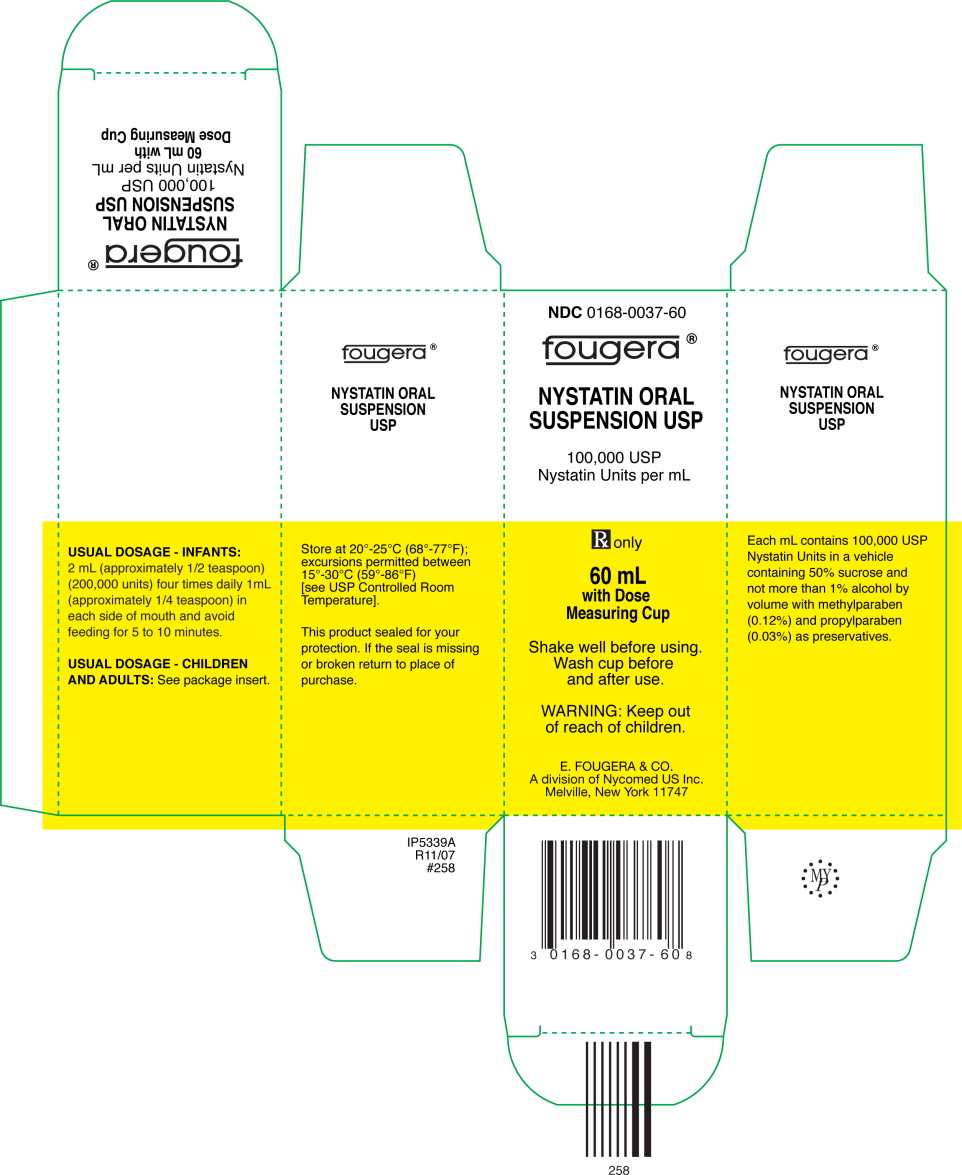
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 60ML CONTAINER
NDC: 0168-0037-60
FOUGERA®
NYSTATIN ORAL
SUSPENSION USP
100,000 USP Nystatin Units per mL
Rx only
60mL
-
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 60ML CARTON
NDC: 0168-0037-60
FOUGERA®
NYSTATIN ORAL
SUSPENSION USP
100,000 USP
Nystatin Units per mL
Rx only
60mL
with Dose
Measuring Cup
Shake well before using.
Wash cup before
and after use.
WARNING: Keep out
of reach of children.
-
INGREDIENTS AND APPEARANCE
NYSTATIN
nystatin suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0168-0037 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength nystatin (UNII: BDF1O1C72E) (nystatin - UNII:BDF1O1C72E) nystatin 100000 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength alcohol (UNII: 3K9958V90M) sodium phosphate, dibasic (UNII: GR686LBA74) glycerin (UNII: PDC6A3C0OX) water (UNII: 059QF0KO0R) silicon dioxide (UNII: ETJ7Z6XBU4) sucrose (UNII: C151H8M554) methylparaben (UNII: A2I8C7HI9T) propylparaben (UNII: Z8IX2SC1OH) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0168-0037-60 1 in 1 CARTON 1 60 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA062517 06/07/1984 Labeler - E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. (043838424) Establishment Name Address ID/FEI Business Operations Fougera Pharmaceuticals Inc. 043838424 ANALYSIS(0168-0037) Establishment Name Address ID/FEI Business Operations Fougera Pharmaceuticals Inc. 174491316 MANUFACTURE(0168-0037)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.