CONTEPO- fosfomycin disodium injection, powder, for solution
Contepo by
Drug Labeling and Warnings
Contepo by is a Prescription medication manufactured, distributed, or labeled by Meitheal Pharmaceuticals Inc., Fisiopharma, s.r.l, Ercros, S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CONTEPO safely and effectively. See full prescribing information for CONTEPO.
CONTEPO (fosfomycin) for injection, for intravenous use
Initial U.S. Approval: 1996INDICATIONS AND USAGE
CONTEPO is an epoxide antibacterial indicated for the treatment of patients 18 years of age and older with complicated urinary tract infections (cUTI) including acute pyelonephritis caused by susceptible isolates of Escherichia coli and Klebsiella pneumoniae. (1.1)
Usage to Reduce Development of Drug-Resistant Bacteria
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CONTEPO and other antibacterial drugs, CONTEPO should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. (1.2)
DOSAGE AND ADMINISTRATION
- Administer CONTEPO, 6 grams every 8 hours by intravenous (IV) infusion over 1 hour for up to 14 days, in patients 18 years of age or older with an estimated creatinine clearance (CLcr) greater than 50 mL/min. (2.1)
- The recommended dosage in patients 18 years of age and older with an estimated CLcr of 50 mL/min or less is presented in the table below. (2.2)
aCLcr estimated using Cockcroft-Gault Equation.
bAll doses of CONTEPO are administered by intravenous infusion over 1 hour.
Estimated CLcr (mL/min)a Loading Doseb Maintenance Dosageb Dose Frequency 41-50 6 grams 4 grams Every 8 hours 31-40 6 grams 3 grams Every 8 hours 21-30 6 grams 5 grams Every 24 hours 11-20 6 grams 3 grams Every 24 hours - Approximately 60% to 80% of the fosfomycin dose is cleared from the body by hemodialysis. Administer CONTEPO after hemodialysis on hemodialysis days. (2.2)
DOSAGE FORMS AND STRENGTHS
CONTEPO for Injection contains 6 grams of fosfomycin as a sterile powder in single-dose vials for reconstitution and further dilution. (3)
CONTRAINDICATIONS
CONTEPO is contraindicated in patients with known serious hypersensitivity to fosfomycin, or any of the excipients. (4)
WARNINGS AND PRECAUTIONS
- Serum Electrolyte Abnormalities: CONTEPO contains 1980 mg sodium in each vial. The high sodium load associated with the use of CONTEPO may result in changes in serum electrolytes, such as increased levels of sodium and decreased levels of potassium, calcium and phosphorus. A low-sodium diet is recommended during CONTEPO treatment. Monitor serum electrolyte levels and fluid status during CONTEPO treatment. Monitor signs and symptoms of edema, particularly in patients with cardiac insufficiency, renal impairment, cirrhosis, hypertension, hyperaldosteronism, hypernatremia or pulmonary edema. (5.1)
- QT Prolongation: CONTEPO has been shown to prolong the QT interval in some patients in the clinical trial. Avoid CONTEPO in patients with known prolongation of the QT interval or ventricular arrhythmias, including a history of torsade de pointes. (5.2, 7.1)
- Increased Transaminase Levels: Increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were reported in the clinical trial. Monitor hepatic enzymes during CONTEPO treatment. (5.3)
- Hypersensitivity Reactions: Reactions such as rash, urticaria and anaphylaxis were reported with the use of CONTEPO. Before initiating therapy with CONTEPO, it is important to inquire about previous hypersensitivity reactions to oral or parenteral fosfomycin. If an allergic reaction occurs, discontinue CONTEPO immediately. (5.4)
- Neutropenia Including Agranulocytosis: Neutropenia, including agranulocytosis, has occurred in patients receiving IV fosfomycin treatment. Monitor complete blood count during CONTEPO therapy. If such reactions occur, discontinue CONTEPO and institute appropriate treatment (5.5).
- Clostridioides difficile-Associated Diarrhea (CDAD): This has been reported with nearly all systemic antibacterial agents, including CONTEPO. Evaluate patients if diarrhea occurs. (5.6)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2%) are transaminase elevations, hypokalemia, neutropenia, nausea, vomiting, diarrhea, hypocalcemia, hypernatremia, headache, and hypophosphatemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Meitheal Pharmaceuticals Inc. at 1-844-824-8426 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding not recommended during treatment and 24 hours after the last dose (8.2). (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Complicated Urinary Tract Infections (cUTI), including Acute Pyelonephritis
1.2 Usage to Reduce Development of Drug-Resistant Bacteria
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Dosage in Patients (18 Years of Age and Older) with Renal Impairment
2.3 Preparation of Diluted Solutions of CONTEPO
2.4 Stability of CONTEPO Solution in Intravenous Fluids
2.5 Drug Incompatibility
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serum Electrolyte Abnormalities
5.2 QT Prolongation
5.3 Increased Transaminase Levels
5.4 Hypersensitivity Reactions
5.5 Neutropenia Including Agranulocytosis
5.6 Clostridioides difficile-Associated Diarrhea
5.7 Development of Drug-resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs that prolong QT interval
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Complicated Urinary Tract Infections, Including Acute Pyelonephritis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Complicated Urinary Tract Infections (cUTI), including Acute Pyelonephritis
CONTEPO is indicated for the treatment of patients 18 years and older with complicated urinary tract infections (cUTI), including acute pyelonephritis, caused by susceptible isolates of Escherichia coli and Klebsiella pneumoniae.
1.2 Usage to Reduce Development of Drug-Resistant Bacteria
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CONTEPO and other antibacterial drugs, CONTEPO should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of CONTEPO is 6 grams administered every 8 hours by intravenous (IV) infusion over 1 hour in patients 18 years of age or older with an estimated creatinine clearance (CLcr) greater than 50 mL/min. The duration of therapy is up to 14 days and should be guided by the severity of infection and the patient's clinical status. During treatment, different dosage recommendations may be required based on change in estimated CLcr [see Dosage and Administration (2.2)].
2.2 Recommended Dosage in Patients (18 Years of Age and Older) with Renal Impairment
The recommended dosage of CONTEPO in patients 18 years of age and older with an estimated CLcr of 50 mL/min or less is presented in Table 1. Monitor estimated CLcr and adjust the dosage of CONTEPO accordingly [see Warnings and Precautions (5.1), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Table 1 Dosage of CONTEPO in Patients (18 Years of Age and Older) with Renal Impairment a CLcr estimated by Cockcroft-Gault Equation.
bAll doses of CONTEPO are administered by IV infusion over 1 hour.
Estimated CLcr (mL/min)a Loading Doseb Maintenance Dosageb Dose Frequency 41-50 6 grams 4 grams Every 8 hours 31-40 6 grams 3 grams Every 8 hours 21-30 6 grams 5 grams Every 24 hours 11-20 6 grams 3 grams Every 24 hours Approximately 60 to 80 % of the fosfomycin dose is cleared from the body by hemodialysis. Administer CONTEPO after hemodialysis on hemodialysis days.
2.3 Preparation of Diluted Solutions of CONTEPO
Preparation
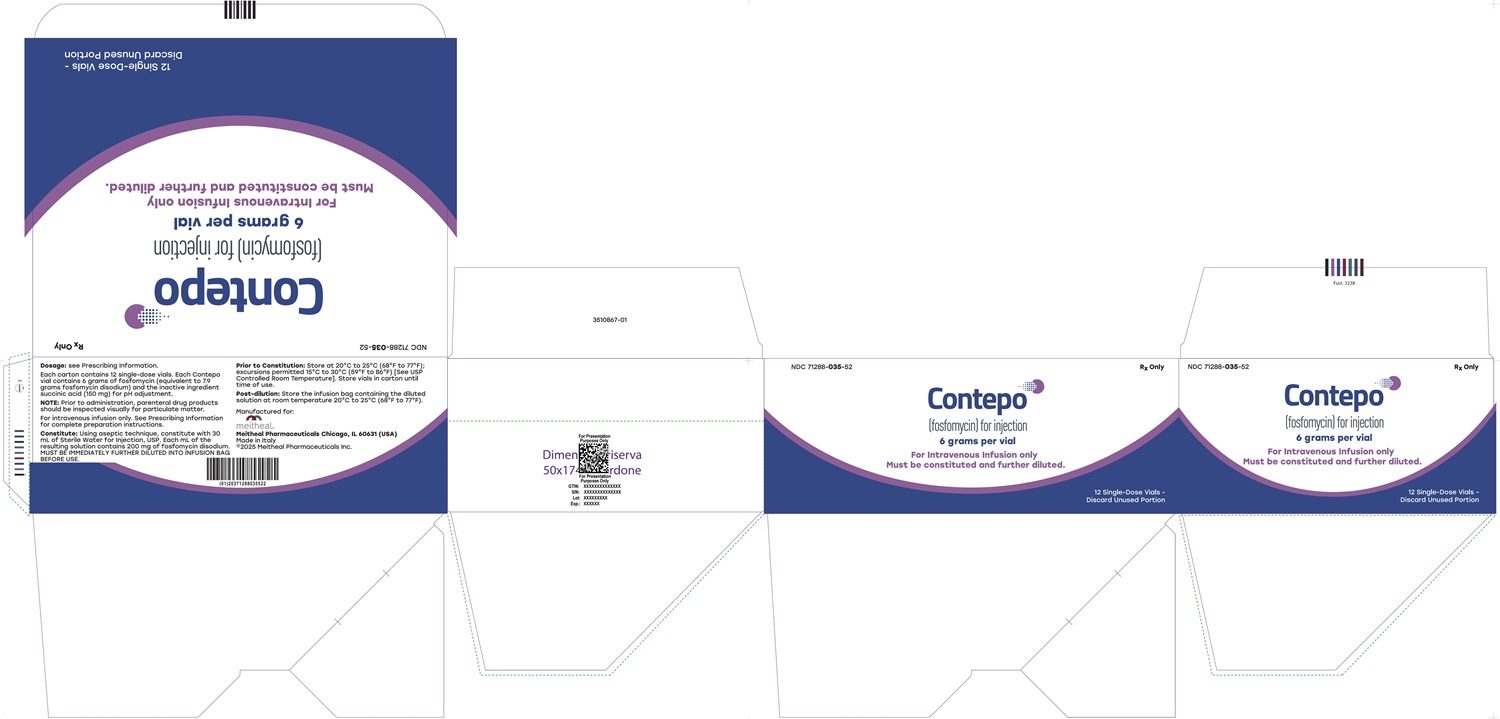
CONTEPO is supplied as a dry powder in a single-dose vial that must be constituted and further diluted prior to intravenous infusion as described below. CONTEPO does not contain preservatives. Aseptic technique must be used for constitution and dilution prior to IV infusion.
- Constitute the vial with 30 mL of Sterile Water for Injection, USP and gently mix to completely dissolve contents. A slight degree of warming occurs when the powder is dissolved. The constituted solution should appear clear and colorless. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The constituted solution is not for direct injection and must be further diluted immediately with Sterile Water for Injection, USP [see Dosage and Administration (2.4)] before intravenous infusion.
- To prepare the infusion solution, first remove 80 mL from a 250 mL intravenous bag of Sterile Water for Injection, USP for infusion so that it contains approximately 170 mL.
- Then add the required volume of constituted solution to the infusion bag according to Table 2. Discard unused portion. The constituted and further diluted solution of CONTEPO has a pH of 7.4 to 7.8.
Table 2 Preparation of CONTEPO Doses CONTEPO Dose Volume to Withdraw from Constituted Vial Volume of Final Infusion Bag
(Approximate)Final Infusion Concentration of CONTEPO
(Approximate)6 grams 32.6 mL
(Entire Contents)202 mL 30 mg/mL 5 grams 27 mL 197 mL 25 mg/mL 4 grams 21.5 mL 192 mL 20 mg/mL 3 grams 16 mL 186 mL 15 mg/mL 2.4 Stability of CONTEPO Solution in Intravenous Fluids
Because CONTEPO contains a high sodium content (1,980 mg per vial) [see Warnings and Precautions (5.2)], the infusion solution for dilution is Sterile Water for Injection, USP. After dilution, CONTEPO solution for administration is stable for 16 hours at room temperature (20°C to 25°C) at a concentration of 15 mg/mL to 30 mg/mL in Sterile Water for Injection, USP.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
CONTEPO is contraindicated in patients with known serious hypersensitivity to fosfomycin, or any of the excipients [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serum Electrolyte Abnormalities
CONTEPO contains 1,980 mg of sodium in each vial. The high sodium load associated with the use of CONTEPO may result in changes in serum electrolytes, such as increased levels of serum sodium and decreased levels of potassium, calcium, and phosphorous. Electrolyte disturbances, such as hypokalemia and hypocalcemia, may potentiate cardiac effects, including QT prolongation [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)].
In Trial 1, a comparator-controlled clinical trial in patients with cUTI, hypokalemia, hypernatremia, hypophosphatemia, and hypocalcemia occurred more frequently in CONTEPO-treated patients compared with piperacillin/tazobactam-treated patients [see Adverse Reactions (6.1)]. Hypokalemia with serum potassium less than 3.0 mEq/L and/or requiring potassium supplementation occurred in 9.9% of patients receiving CONTEPO and 1.7% of patients receiving piperacillin/tazobactam. Hypophosphatemia with serum phosphate concentrations less than 1.0 mg/dL occurred in 2.1% of patients receiving CONTEPO and none of the patients receiving piperacillin/tazobactam. Hypernatremia with serum sodium greater than 150 mEq/L occurred in 3.4% of patients receiving CONTEPO and 0.9% of patients receiving piperacillin/tazobactam. Hypocalcemia with corrected serum calcium less than 8.0 mg/dL occurred in 3.9% of patients receiving CONTEPO and 2.6% of patients receiving piperacillin/tazobactam.
A low-sodium diet is recommended during CONTEPO treatment. Minimize administration of drugs containing sodium. Monitor serum electrolyte levels (sodium, potassium, calcium, magnesium, and phosphorus) and fluid status during treatment with CONTEPO. Electrolyte supplementation may be necessary in some cases. Monitor for signs of edema, particularly in patients who should restrict their sodium intake or are prone to fluid overload (e.g., cardiac insufficiency, renal impairment, cirrhosis, hypertension, hyperaldosteronism, hypernatremia or pulmonary edema) [see Adverse Reactions (6.1)].
5.2 QT Prolongation
CONTEPO has been shown to prolong the QT interval in some patients. QT prolongation can lead to development of torsade de pointes-type ventricular tachycardia with the risk increasing as the degree of prolongation increases. In Trial 1, 3.6% of patients treated with CONTEPO had > 60 msec increase in QTcF interval from baseline and 1% of patients had QTcF > 480 msec. Torsade de pointes has been reported during postmarketing experience with fosfomycin [see Adverse Reactions (6.2)]. The risk of QT prolongation may increase in patients with electrolyte abnormalities (such as hypokalemia and hypocalcemia), patients with conditions that have potential to cause electrolyte imbalance (such as renal impairment), or patients taking other drugs affecting electrolyte balance or QT prolongation. Avoid CONTEPO in patients with known QT prolongation or ventricular arrhythmias, including a history of torsade de pointes. Monitor electrolytes during treatment with CONTEPO [see Warnings and Precautions (5.1), Drug Interactions (7.1) and Clinical Pharmacology (12.2)].
5.3 Increased Transaminase Levels
In Trial 1, increases in transaminases occurred more frequently in CONTEPO-treated patients compared to piperacillin/tazobactam-treated patients. Transaminases (Alanine Aminotransferase (ALT) or Aspartate Aminotransferase (AST) were elevated ≥ 3x upper limit of normal (ULN) in 10.3% of patients receiving CONTEPO and 4.8% of patients receiving piperacillin/tazobactam. Transaminase elevations were asymptomatic and reversible. No concurrent increases in serum bilirubin or alkaline phosphatase were observed. No patients met Hy's Law criteria, and no patients discontinued CONTEPO therapy because of treatment-related transaminase elevation [see Adverse Reactions (6.1)]. Monitor hepatic enzymes during CONTEPO treatment.
5.4 Hypersensitivity Reactions
CONTEPO is contraindicated in patients with known serious hypersensitivity to fosfomycin, or any of the excipients [see Contraindications (4)]. Hypersensitivity reactions, such as rash and urticaria, were reported in patients treated with CONTEPO in Trial 1. Anaphylaxis has been reported postmarketing [see Adverse Reactions (6.2)]. Before initiating therapy with CONTEPO, it is important to inquire about previous hypersensitivity reactions to oral or parenteral fosfomycin. If an allergic reaction to CONTEPO occurs, discontinue the drug immediately.
5.5 Neutropenia Including Agranulocytosis
Neutropenia has been reported in patients receiving IV fosfomycin therapy. In Trial 1, 6.4% of patients in the CONTEPO arm developed neutropenia (absolute neutrophil count less than 1500 cells/mm3) as compared to 3.9% in the piperacillin/tazobactam comparator arm. In the post marketing setting, neutropenia cases progressing to agranulocytosis have been reported with IV fosfomycin therapy. Monitor complete blood counts during CONTEPO therapy particularly in patients with pre-existing conditions or patients receiving concomitant drugs that may predispose to bone marrow suppression. Discontinue CONTEPO if neutropenia occurs and consider alternative therapies.
5.6 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all systemic antibacterial agents, including CONTEPO, and may range in severity from mild to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after administration of antibacterial agents.
If CDAD is confirmed, discontinue antibacterials not directed against C. difficile, if possible. Manage fluid and electrolyte levels as appropriate, supplement protein intake, monitor antibacterial treatment of C. difficile, and institute surgical evaluation as clinically indicated.
5.7 Development of Drug-resistant Bacteria
Prescribing CONTEPO in the absence of a proven or strongly suspected bacterial infection indication is unlikely to provide benefit to the patient and increases the risk of the development of drug resistant bacteria [see Indications and Usage (1.2)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in the Warnings and Precautions section:
- Serum Electrolyte Abnormalities [see Warnings and Precautions (5.1)]
- QT Prolongation [see Warnings and Precautions (5.2)]
- Increased Transaminase Levels [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Neutropenia Including Agranulocytosis [see Warnings and Precautions (5.5)]
- Clostridioides difficile-Associated Diarrhea [see Warnings and Precautions (5.6)]
- Development of Drug-Resistant Bacteria [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
CONTEPO was evaluated in a comparator-controlled clinical trial (Trial 1) in patients with cUTI, including acute pyelonephritis, which included 233 patients treated with CONTEPO and 231 treated with comparator (piperacillin/tazobactam 4.5 g every 8 hours) for 7 days, allowing bacteremic patients to receive up to 14 days. No switch to oral antibacterial drugs was allowed. The median age of treated patients was 54 years (range 18-89 years) and 64% were female. All patients were white (100%). Patients (99%) were predominantly enrolled in Eastern Europe. Concomitant bacteremia was identified in 9% of patients at baseline.
Serious Adverse Reactions and Adverse Reactions Leading to Discontinuation
Serious adverse reactions occurred in 2.1% (5/233) CONTEPO and 2.6% (6/231) piperacillin/tazobactam-treated patients, respectively. Treatment was discontinued due to adverse reactions in 3% (7/233) of patients receiving CONTEPO and in 2.6% (7/231) of patients receiving piperacillin/tazobactam. The most common adverse reactions resulting in discontinuation of CONTEPO were gastrointestinal disorders (nausea, vomiting, and abdominal pain) in 1.3% (3/233) of patients. No deaths occurred in the clinical trial.
Common Adverse Reactions
Table 3 lists adverse reactions occurring in 2% or greater of patients receiving CONTEPO in Trial 1. These adverse reactions were reversible upon completion of therapy.
Table 3 Adverse Reactions Occurring in 2% or Greater of Patients with cUTI Receiving CONTEPO in Trial 1 aTransaminase elevations include increased ALT and AST ≥3x ULN.
bNeutropenia includes absolute neutrophil count <1500 cells/mm3
Adverse Reaction CONTEPO
N=233
%Piperacillin/Tazobactam
N=231
%Gastrointestinal Disorders Nausea 4.3 1.3 Diarrhea 3.9 4.8 Vomiting 3.9 0.4 Laboratory Investigations Transaminase elevations a 10.3 4.8 Hypokalemia 9.9 1.7 Hypophosphatemia 2.1 0.0 Hypocalcemia 3.9 2.6 Hypernatremia 3.4 0.9 Blood and Lymphatic System Disorders Neutropeniab 6.4 3.9 Nervous System Disorders Headache 2.6 2.2 Adverse Reactions Occurring in < 2% of Patients Receiving CONTEPO in Trial 1:
Blood and lymphatic system disorders: anemia, thrombocytopenia
Cardiac disorders: atrial fibrillation, palpitations, tachycardia, heart failure
Ear and labyrinth disorders: hearing loss
Gastrointestinal disorders: constipation
General disorders and administration site conditions: asthenia, infusion site reactions, peripheral edema
Hepatobiliary disorders: hepatic steatosis, hepatomegaly
Infections and infestations: vaginal infection, vaginitis
Investigations: increase creatinine kinase
Metabolism and nutritional disorders: hyperglycemia
Nervous system disorders: dysgeusia, syncope
Respiratory, thoracic, and mediastinal disorders: dyspnea
Skin and subcutaneous disorders: urticaria, rash, pruritis
6.2 Postmarketing Experience
The following additional adverse reactions were not reported with CONTEPO-treated patients in Trial 1 but have been identified with the use of oral fosfomycin tromethamine or during use of intravenous fosfomycin sodium outside of the United States for various indications and dosing regimens. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Blood and lymphatic system disorders: aplastic anemia, eosinophilia, agranulocytosis, leukopenia, pancytopenia
Cardiac disorders: torsade de pointes
Ear and labyrinth disorders: vertigo
Eye disorders: visual impairment
Gastrointestinal disorders: dyspepsia, C. difficile-associated diarrhea and colitis, toxic megacolon
Hepatobiliary disorders: alkaline phosphatase increased, cholestatic hepatitis, icterus, hepatic necrosis
Immune system disorders: anaphylaxis
Metabolism and nutrition disorders: decreased appetite
Nervous system disorders: cerebral edema, dizziness, optic neuritis
Psychiatric disorders: confusion
Respiratory, thoracic, and mediastinal disorders: asthma attack, pulmonary edema
Skin and subcutaneous tissue disorders: angioedema, facial edema
Vascular disorders: hypertension
-
7 DRUG INTERACTIONS
7.1 Drugs that prolong QT interval
Co-administration of CONTEPO with other drugs known to prolong the QT interval may increase the risk for ventricular arrhythmia. Avoid co-administration of CONTEPO with drugs known to prolong the QT interval, such as class IA or class III antiarrhythmic medications, tricyclic antidepressants, macrolides, and antipsychotics [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from observational studies and pharmacovigilance reports with fosfomycin use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Fosfomycin crosses the placental barrier. There are no animal data that meet current standards for nonclinical developmental toxicity studies. However, some reproductive toxicity data are available from published literature. Intravenous or intraperitoneal fosfomycin-sodium did not cause malformations in rabbits or rats, respectively, but showed evidence of fetotoxicity (see Data). The clinical relevance of these animal data is uncertain.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Fosfomycin crosses the placenta in rats and rabbits. In rats administered intraperitoneal fosfomycin sodium on Days 7 to 17 of gestation (during organogenesis), there were increased numbers of dead or resorbed fetuses at 1500 mg/kg (approximately 0.8 times the recommended human dose of 18 g/day, based on body surface area comparisons), a dose associated with maternal toxicity . Rabbits were administered intravenous fosfomycin sodium on Days 6 to 18 of gestation (during organogenesis) at doses 800 mg/kg (approximately 0.9 times the recommended human dose). No malformations were observed in rabbits or rats after intravenous or intraperitoneal fosfomycin sodium, respectively.
In a pre- and post-natal developmental study in rats (dosed intraperitoneally with fosfomycin tromethamine between gestational day 6 and postnatal Day 21), no effects were observed in first-generation offspring at doses up to 1000 mg/kg, about 0.5 times the clinical dose, based on body surface area comparisons. Survival and postnatal body growth also were normal in the second-generation offspring at all doses.
8.2 Lactation
Risk Summary
Fosfomycin is present in human milk. There are no data on the effects of fosfomycin on the breastfed child or on milk production. Because of the potential for adverse reactions in a breastfed infant, advise patients not to breastfeed during treatment with CONTEPO and for 24 hours after the last dose.
8.4 Pediatric Use
Safety and effectiveness of CONTEPO in patients less than 18 years of age has not been established.
8.5 Geriatric Use
Of the 233 patients treated with CONTEPO in Trial 1, 32% (74/233) were 65 years of age and older, including 11.2% (26/233) who were 75 years of age and older. For patients included in the microbiological modified intent-to-treat (mMITT) population, lower overall success rates were observed for CONTEPO-treated and piperacillin/tazobactam-treated patients ≥ 65 years of age (53.6% and 43.9%, respectively) as compared to patients < 65 years (68.4% and 61.6%, respectively).
For patients ≥ 65 years of age, the incidence rate of adverse reactions was 39.2% (29/74) in CONTEPO-treated patients and 28.6% (22/77) in the piperacillin/tazobactam-treated patients. For patients <65 years of age, the incidence rate of adverse reactions was 43.4% (69/159) in CONTEPO-treated patients and 33.8% (52/154) in the piperacillin/tazobactam-treated patients.
Because elderly patients are more likely to have decreased cardiac and renal function, care should be taken in dose selection, and electrolytes, fluid status, and renal function should be monitored. Dosage adjustment in elderly patients should take into account renal function [see Dosage and Administration (2.2, 2.3, 2.4) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Dosage adjustment is required for patients with estimated CLcr 50 mL/min or less. Monitor estimated CLcr and adjust the dosage of CONTEPO accordingly [see Dosage and Administration (2.2)].
In patients requiring hemodialysis, CONTEPO should be administered after hemodialysis on hemodialysis days [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
To date, no cases of accidental overdose with clinically relevant intolerances have been reported. In the event of an overdose, the patient must be monitored and treated symptomatically. Fosfomycin is cleared from the body by hemodialysis, during which patients with end stage renal disease have an increased mean fosfomycin elimination half-life to approximately 4 hours.
-
11 DESCRIPTION
CONTEPO (fosfomycin) for injection, for intravenous use, contains fosfomycin disodium, an epoxide antibacterial drug.
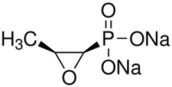
Fosfomycin disodium is a powder with the chemical name of disodium [(2R,3S)-3-methyloxiran-2-yl]-dioxido-oxophosphorane, an empirical formula of C3H5Na2O4P and molecular weight of 182.
Figure 1 Chemical Structure of Fosfomycin Disodium
Each CONTEPO for Injection single-dose vial contains white to almost white sterile powder with 6 grams of fosfomycin (equivalent to 7.9 grams fosfomycin disodium) and the inactive ingredient succinic acid (150 mg) for pH adjustment. It is intended for constitution and further dilution prior to intravenous infusion . Each gram of fosfomycin disodium contains 330 mg of sodium (i.e., each vial contains 1,980 mg of sodium).
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
The ratio of the unbound plasma fosfomycin AUC to MIC against the infecting organism has been shown to correlate with activity in animal models of infection.
Cardiac Electrophysiology
The effect of CONTEPO on 12-lead electrocardiogram parameters was evaluated in a Phase 1 randomized, placebo and positive controlled, double-blind, single-dose crossover study in 36 healthy adult subjects. At single doses of 6 grams and 12 grams (2 times the maximum single recommended dosage), CONTEPO demonstrated a dose dependent increase in QTcF. Mean placebo-corrected QTcF change from baseline was 8.3 msec (90% CI: 5.39 to 11.30) and 17.0 msec (90% CI: 14.06 to 20.01) for single doses of 6 grams and 12 grams, respectively, compared to 13.4 msec (90% CI: 10.48 to 16.39) observed with the active control, oral moxifloxacin. There were no subjects receiving CONTEPO with QTcF change from baseline greater than 60 msec or a QTcF greater than 480 msec [see Warnings and Precautions (5.2)]. A 1-hour CONTEPO infusion of the studied doses did not have a clinically meaningful effect on heart rate or on cardiac conduction, i.e., the PR and QRS interval.
12.3 Pharmacokinetics
The mean pharmacokinetic parameters of fosfomycin in healthy adults with normal renal function after single doses of 6 grams administered as 1-hour IV infusion are summarized along with additional pharmacokinetic information in Table 4.
Table 4 Exposure Parameter Estimates (Mean ± SD)a Following Single Dose of 6 grams Fosfomycin Administered as 1-hour IV Infusion in Healthy Adults with Normal Renal Function a Based on non-compartmental analysis of PK data
Cmax (mcg/mL) 228 ± 43 AUC0-inf (mcg∙hr/mL) 734 ± 134 Dose Proportionality Fosfomycin Cmax and AUC0-inf increase proportionally with dose Distribution Volume of Distribution (L) 27 ± 5.2 Protein Binding Fosfomycin is not bound to plasma proteins Elimination Half-Life (h) 2.8 ± 0.6 Total Clearance (L/h) 8.5 ± 1.7 Metabolism Fosfomycin is not metabolized. Excretion Urine: 70% of dose is excreted unchanged at 12 hours; 74-80% of dose is excreted unchanged at 48 hours Specific Populations
No clinically significant differences in the pharmacokinetics of fosfomycin based on sex, body weight/body surface area, race/ethnicity or age (18 to 89 years of age, when adjusted for renal function) were identified.
Patients with Renal Impairment
Dosage adjustment is required for patients whose creatinine clearance is 50 mL/min or less [see Dosage and Administration (2.2), Use in Specific Populations (8.6)]. When fosfomycin is administered prior to hemodialysis in patients on periodic or chronic hemodialysis, 61-79% of the fosfomycin dose is removed.
Patients with Hepatic Impairment
CONTEPO is not metabolized through the liver. The effect of hepatic impairment on the pharmacokinetics of CONTEPO is unknown. Monitoring fluid overload and electrolyte abnormalities is recommended for patients with severe hepatic impairment [see Warnings and Precautions (5.1), Use in Specific Populations (8.7)].
Drug Interaction Studies
In Vitro Studies
Fosfomycin at clinically relevant concentrations does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5. Fosfomycin does not induce CYP1A2, CYP2B6, and CYP3A4. Fosfomycin is not a substrate for P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1 or MATE2-K. Fosfomycin inhibits MATE1 and MATE2-K with the observed IC50 values of 30.0 mM (4142 mcg/mL) and 56.4 mM (7787 mcg/mL), respectively. Fosfomycin does not inhibit P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, and OCT2.
12.4 Microbiology
Mechanism of Action
Fosfomycin is an epoxide antibacterial drug that disrupts bacterial cell wall synthesis by covalently binding and inhibiting phosphoenolpyruvate transferase (MurA), thereby blocking the synthesis of peptidoglycan. Fosfomycin is bactericidal against Enterobacterales. Transport of fosfomycin into the bacterial cell occurs via two different transport systems, glycerol-3-phosphate (GlpT) and/or hexose-6-phosphate (UhpT).
Resistance
Resistance to fosfomycin may occur by chromosomal mutations leading to alterations of bacterial transport systems and/or modification of the fosfomycin binding site in MurA (Cys115). Spontaneous mutations conferring various levels of fosfomycin resistance in E. coli and other Enterobacterales in vitro have been shown to occur in the structural transport genes (glpT and uhpT), transport regulatory genes (uhpA, uhpB, and uhpC) and genes involved in cAMP synthesis (cyaA, ptsI), all causing a decrease in fosfomycin uptake. Diminished activity of both transport systems is also evident when inactivation of cAMP-CRP occurs.
Plasmid-borne resistance mechanisms may result in enzymatic inactivation of fosfomycin by binding to glutathione, or by cleavage of the carbon-phosphorus-bond in the fosfomycin molecule. The enzymes responsible for this type of resistance are fosfomycin hydrolyzing enzymes (FosA, FosB, FosX) and fosfomycin kinases (FomA, FomB and FosC).
Resistance to fosfomycin due to spontaneous mutations occurs at frequencies between 10-7 to 10-9 for E. coli and 10-5 to 10-8 for K. pneumoniae at 4 times the fosfomycin Minimum Inhibitory Concentration (MIC).
There is no known cross-resistance to other antibacterial drug classes.
Interaction With Other Antimicrobials
In vitro studies have not demonstrated antagonism between CONTEPO and the following antibacterial drugs: amikacin, gentamicin, aztreonam, ceftazidime, ceftriaxone, piperacillin/tazobactam, meropenem, levofloxacin, tigecycline, minocycline, linezolid, rifampin, trimethoprim-sulfamethoxazole, vancomycin, penicillin and colistin. The clinical significance of these findings is unknown.
Animal Infection Models
Fosfomycin demonstrated activity in neutropenic thigh infection models caused by either E. coli (KPC and NDM producing) or K. pneumoniae (KPC and VIM producing) isolates.
Antimicrobial Activity
CONTEPO has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1.1)].
Aerobic bacteria
Gram-negative bacteria
Escherichia coli
Klebsiella pneumoniae
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for CONTEPO against isolates of similar genus or organism group. However, the efficacy of CONTEPO in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Aerobic bacteria
Gram-negative bacteria
Citrobacter koseri
Enterobacter aerogenes
Klebsiella oxytoca
Proteus mirabilis
Serratia marcescens
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
Fosfomycin did not show evidence of mutagenic activity in standard tests that included bacterial reverse mutation assay, chromosomal aberration assay with Chinese hamster lung fibroblast cells and human peripheral blood lymphocytes, and the mouse bone marrow micronucleus assay.
Impairment of Fertility
In a fertility study, male and female rats were dosed intraperitoneally with fosfomycin disodium (for 7 or 14 days, respectively, before mating, for 14 days during cohabitation, and in mated females up to gestation day 7 at doses of 125, 250, 750 or 1500 mg/kg. Maternal toxicity (mortality) was observed at the 1500 mg/kg dose (about 0.8 times the clinical dose based on body surface area comparisons) along with increases in the number of dead or resorbed fetuses and a reduction in the number of live fetuses. There were no effects on fertility at 750 mg/kg (approximately 0.4 times the clinical dose).
-
14 CLINICAL STUDIES
14.1 Complicated Urinary Tract Infections, Including Acute Pyelonephritis
A total of 464 adults hospitalized with cUTI (including acute pyelonephritis) were randomized and received study medications in a multinational, double-blind study (Trial 1, NCT02753946) comparing CONTEPO 6 g intravenously every 8 hours to piperacillin/tazobactam 4.5 g intravenously every 8 hours for 7 days of therapy. Treatment was allowed for up to 14 days in bacteremic patients. The study did not include a switch to oral antibacterial drugs.
The microbiological modified intent-to-treat (mMITT) population included all patients who received study medication and had at least 1 baseline uropathogen, excluding patients with organisms resistant to study drugs at baseline. This population consisted of 339 patients with cUTI, including 184 patients (54%) with acute pyelonephritis. The median age was 53 years and 64% were female. Concomitant bacteremia was identified in 9% of patients at baseline, and 32% of patients met 2 or more systemic inflammatory response syndrome (SIRS) criteria at baseline.
Overall success was defined as clinical cure plus microbiological eradication (baseline uropathogens with growth in urine ≥105 CFU/mL were reduced to <104 CFU/mL) at the test-of-cure (TOC) visit, 19 (+2) days after randomization. CONTEPO demonstrated efficacy with regard to the overall response at the TOC visit in the mMITT population (Table 5). A sensitivity analysis for overall success at the TOC visit using a threshold of 103 CFU/ml for microbiological eradication demonstrated similar efficacy results.
In the mMITT population, the overall success rates at TOC in patients with concurrent bacteremia were 9/19 (47.4%) for CONTEPO-treated patients and 5/13 (38.5%) in the piperacillin/tazobactam-treated patients.
Overall response rates at the TOC visit by pathogen in the mMITT population is presented in Table 6.
Table 5 Overall Response Rates at Test-of-Cure Visit in cUTI Patients in Trial 1 (mMITT Population) 1 Patients with an organism resistant to study drugs at baseline were to be excluded. All of the patients excluded
(14 in CONTEPO arm, 9 in piperacillin/tazobactam arm) had an organism resistant to piperacillin/tazobactam.
2 CONTEPO 6 g intravenously every 8 hours
3 Piperacillin/tazobactam 4.5 g every 8 hours
Response 1 CONTEPO2
n/N (%)Piperacillin/
Tazobactam3
n/N (%)Treatment Difference
(95% CI)Overall success 108/170 (63.5) 94/169 (55.6) 7.9 (-3.1, 18.9) Clinical cure 154/170 (90.6) 155/169 (91.7) -1.1 (-7.8, 5.5) Microbiological eradication 110/170 (64.7) 97/169 (57.4) 7.3 (-3.6, 18.3) Table 6 Overall Response Rates at Test-of-Cure Visit by Baseline Pathogen in cUTI Patients in Trial 1 (mMITT Population) 1 Patients with an organism resistant to piperacillin/tazobactam at baseline were excluded
Pathogen1 CONTEPO
n/N (%)Piperacillin/
Tazobactam
n/N (%)Escherichia coli 87/130 (66.9) 75/130 (57.7) Klebsiella pneumoniae 8/18 (44.4) 11/20 (55.0) -
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
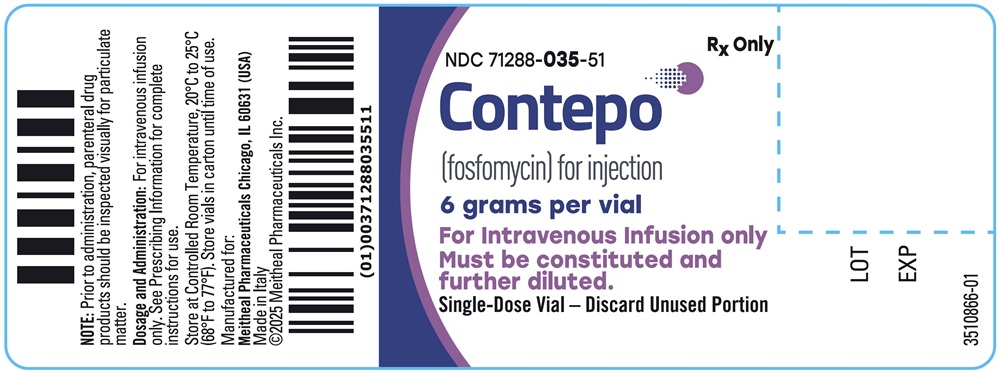
CONTEPO for injection is supplied as a white to almost white sterile powder containing 6 grams of fosfomycin in a single-dose vial. Each gram of fosfomycin disodium contains 330 mg of sodium (i.e., each vial contains 1,980 mg of sodium).
CONTEPO is supplied in a clear Type I glass single-dose vial (NDC: 71288-035-51) with a rubber closure and a twist-off cap. Twelve (12) vials are supplied in each carton (NDC: 71288-035-52).
Storage and Handling
Store CONTEPO vials at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Store CONTEPO in the carton until time of use [see Dosage and Administration (2.4)].
-
17 PATIENT COUNSELING INFORMATION
Electrolyte Abnormalities
Advise patients that a low sodium diet is recommended during CONTEPO treatment because the high sodium load (each vial of CONTEPO contains 1,980 mg sodium) may cause changes in electrolytes or increased edema [see Warnings and Precautions (5.1)]. Blood tests to monitor electrolytes will be required. If peripheral edema develops, tell the patient to contact their health care provider.
Serious Allergic Reactions
Advise patients that allergic reactions, including serious allergic reactions, could occur and that serious reactions require immediate treatment. Ask patient about any previous hypersensitivity reactions to CONTEPO, fosfomycin or other allergens [see Warnings and Precautions (5.2)].
Potentially Serious Diarrhea
Advise patients that diarrhea is a common problem caused by antibacterial drugs, including CONTEPO. Sometimes, frequent watery or bloody diarrhea may occur and may be a sign of a more serious intestinal infection. If severe watery or bloody diarrhea develops, tell patient to contact his or her healthcare provider [see Warnings and Precautions (5.6)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including CONTEPO should only be used to treat bacterial infections. They do not treat viral infections (e.g. the common cold). When CONTEPO is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by CONTEPO or other antibacterial drugs in the future [see Warnings and Precautions (5.7)].
Lactation
Advise females not to breastfeed during treatment with CONTEPO and for 24 hours after the final dose [see Use in Specific Populations (8.2)].
Manufactured for Meitheal Pharmaceuticals
Chicago, IL 60631 (USA)
Made in Italy
©2025 Meitheal Pharmaceuticals Inc.CONTEPO is a trademark of Meitheal Pharmaceuticals, Inc.
All rights reserved3510868-02
- PRINCIPAL DISPLAY PANEL - CONTEPO 6 grams per vial Container Label
- PRINCIPAL DISPLAY PANEL - CONTEPO 6 grams per vial Carton Label
-
INGREDIENTS AND APPEARANCE
CONTEPO
fosfomycin disodium injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71288-035 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSFOMYCIN SODIUM (UNII: 97MMO19FNO) (FOSFOMYCIN - UNII:2N81MY12TE) FOSFOMYCIN 6 g Inactive Ingredients Ingredient Name Strength succinic acid (UNII: AB6MNQ6J6L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71288-035-52 12 in 1 CARTON 10/22/2025 1 NDC: 71288-035-51 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212271 10/22/2025 Labeler - Meitheal Pharmaceuticals Inc. (080548348) Registrant - Meitheal Pharmaceuticals Inc. (080548348) Establishment Name Address ID/FEI Business Operations Fisiopharma, s.r.l 441067444 manufacture(71288-035) Establishment Name Address ID/FEI Business Operations Ercros, S.A. 463721500 api manufacture(71288-035)
Trademark Results [Contepo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CONTEPO 98039678 not registered Live/Pending |
Zavante Therapeutics, Inc. 2023-06-13 |
 CONTEPO 90830807 not registered Live/Pending |
Zavante Therapeutics, Inc. 2021-07-15 |
 CONTEPO 88383838 not registered Live/Pending |
Nabriva Therapeutics Ireland DAC 2019-04-12 |
 CONTEPO 87369724 not registered Live/Pending |
NABRIVA THERAPEUTICS US, INC. 2017-03-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.