XIIDRA- lifitegrast solution/ drops
Xiidra by
Drug Labeling and Warnings
Xiidra by is a Prescription medication manufactured, distributed, or labeled by Novartis Pharmaceuticals Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XIIDRA safely and effectively. See full prescribing information for XIIDRA.
XIIDRA ®(lifitegrast ophthalmic solution), for topical ophthalmic use
Initial U.S. Approval: 2016INDICATIONS AND USAGE
Xiidra (lifitegrast ophthalmic solution) 5% is a lymphocyte function-associated antigen-1 (LFA-1) antagonist indicated for the treatment of the signs and symptoms of dry eye disease (DED). ( 1)
DOSAGE AND ADMINISTRATION
One drop twice daily in each eye (approximately 12 hours apart). ( 2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing lifitegrast 50 mg/mL (5%). ( 3)
CONTRAINDICATIONS
Hypersensitivity. ( 4)
ADVERSE REACTIONS
The most common adverse reactions (incidence 5%-25%) following the use of Xiidra were instillation-site irritation, dysgeusia, and decreased visual acuity. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Instill one drop of Xiidra twice daily (approximately 12 hours apart) into each eye using a single-use container. Discard the single-use container immediately after using in each eye.
Contact lenses should be removed prior to the administration of Xiidra and may be reinserted 15 minutes following administration.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity [see Contraindications (4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In five clinical trials of DED conducted with lifitegrast ophthalmic solution, 1401 patients received at least one dose of lifitegrast (1287 of which received lifitegrast 5%). The majority of patients (84%) had less than or equal to 3 months of treatment exposure. One hundred-seventy patients were exposed to lifitegrast for approximately 12 months. The majority of the treated patients were female (77%). The most common adverse reactions reported in 5%-25% of patients were instillation-site irritation, dysgeusia, and reduced visual acuity.
Other adverse reactions reported in 1%-5% of the patients were blurred vision, conjunctival hyperemia, eye irritation, headache, increased lacrimation, eye discharge, eye discomfort, eye pruritus, and sinusitis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Xiidra. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Rare serious cases of hypersensitivity, including anaphylactic reaction, bronchospasm, respiratory distress, pharyngeal edema, swollen tongue, urticaria, allergic conjunctivitis, dyspnea, angioedema, and allergic dermatitis have been reported. Eye swelling and rash have also been reported [see Contraindications (4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Xiidra use in pregnant women to inform any drug-associated risks. Intravenous (IV) administration of lifitegrast to pregnant rats, from premating through gestation day 17, did not produce teratogenicity at clinically relevant systemic exposures. Intravenous administration of lifitegrast to pregnant rabbits during organogenesis produced an increased incidence of omphalocele at the lowest dose tested, 3 mg/kg/day (400-fold the human plasma exposure at the recommended human ophthalmic dose [RHOD], based on the area under the curve [AUC] level). Since human systemic exposure to lifitegrast following ocular administration of Xiidra at the RHOD is low, the applicability of animal findings to the risk of Xiidra use in humans during pregnancy is unclear [see Clinical Pharmacology (12.3)].
Data
Animal Data
Lifitegrast administered daily by IV injection to rats, from premating through gestation day 17, caused an increase in mean pre-implantation loss and an increased incidence of several minor skeletal anomalies at 30 mg/kg/day, representing 5,400-fold the human plasma exposure at the RHOD of Xiidra, based on AUC. No teratogenicity was observed in the rat at 10 mg/kg/day (460-fold the human plasma exposure at the RHOD, based on AUC). In the rabbit, an increased incidence of omphalocele was observed at the lowest dose tested, 3 mg/kg/day (400-fold the human plasma exposure at the RHOD, based on AUC), when administered by IV injection daily from gestation days 7 through 19. A fetal no observed adverse effect level (NOAEL) was not identified in the rabbit.
8.2 Lactation
Risk Summary
There are no data on the presence of lifitegrast in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to lifitegrast from ocular administration is low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for Xiidra and any potential adverse effects on the breastfed child from Xiidra.
-
11 DESCRIPTION
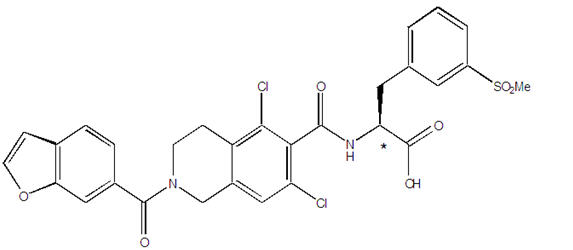
The chemical name for lifitegrast is (S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoic acid. The molecular formula of lifitegrast is C 29H 24Cl 2N 2O 7S and its molecular weight is 615.5 g/mol. The structural formula of lifitegrast is:
*Chiral center
Lifitegrast is a white to off-white powder, which is soluble in water.
Xiidra (lifitegrast ophthalmic solution) 5% is a lymphocyte function-associated antigen-1 (LFA-1) antagonist supplied as a sterile, clear, colorless to slightly brownish-yellow colored, isotonic solution of lifitegrast with a pH of 7.0-8.0, and an osmolality range of 200-330 mOsmol/kg.
Xiidra contains Active:lifitegrast 50 mg/mL; Inactives: sodium chloride, sodium phosphate dibasic anhydrous, sodium thiosulfate pentahydrate, and water for injection. Sodium hydroxide and/or hydrochloric acid (to adjust pH).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lifitegrast binds to the integrin LFA-1, a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular adhesion molecule-1 (ICAM-1). ICAM-1 may be overexpressed in corneal and conjunctival tissues in DED. LFA-1/ICAM-1 interaction can contribute to the formation of an immunological synapse resulting in T-cell activation and migration to target tissues. In vitrostudies demonstrated that lifitegrast may inhibit T-cell adhesion to ICAM-1 in a human T-cell line and may inhibit secretion of inflammatory cytokines in human peripheral blood mononuclear cells. The exact mechanism of action of lifitegrast in DED is not known.
12.3 Pharmacokinetics
In a subset of DED patients (n = 47) enrolled in a Phase 3 trial, the pre-dose (trough) plasma concentrations of lifitegrast were measured after 180 and 360 days of topical ocular dosing (one drop twice daily) with Xiidra (lifitegrast ophthalmic solution) 5%. A total of nine of the 47 patients (19%) had plasma lifitegrast trough concentrations above 0.5 ng/mL (the lower limit of assay quantitation). Trough plasma concentrations that could be quantitated ranged from 0.55 ng/mL to 3.74 ng/mL.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal studies have not been conducted to determine the carcinogenic potential of lifitegrast.
Mutagenesis
Lifitegrast was not mutagenic in the in vitroAmes assay. Lifitegrast was not clastogenic in the in vivomouse micronucleus assay. In an in vitrochromosomal aberration assay using mammalian cells (Chinese hamster ovary cells), lifitegrast was positive at the highest concentration tested, without metabolic activation.
Impairment of Fertility
Lifitegrast administered at IV doses of up to 30 mg/kg/day (5400-fold the human plasma exposure at the RHOD of lifitegrast ophthalmic solution, 5%) had no effect on fertility and reproductive performance in male and female-treated rats.
-
14 CLINICAL STUDIES
The safety and efficacy of lifitegrast for the treatment of DED were assessed in a total of 1181 patients (1067 of which received lifitegrast 5%) in four 12-week, randomized, multi-center, double-masked, vehicle-controlled studies. Patients were randomized to Xiidra or vehicle (placebo) in a 1:1 ratio and dosed twice a day. Use of artificial tears was not allowed during the studies. The mean age was 59 years (range, 19-97 years). The majority of patients were female (76%). Enrollment criteria included minimal signs (i.e., Corneal Fluorescein Staining and non-anesthetized Schirmer Tear Test) and symptoms (i.e., Eye Dryness Score (EDS) and Ocular Discomfort Score) severity scores at baseline.
Effects on Symptoms of Dry Eye Disease
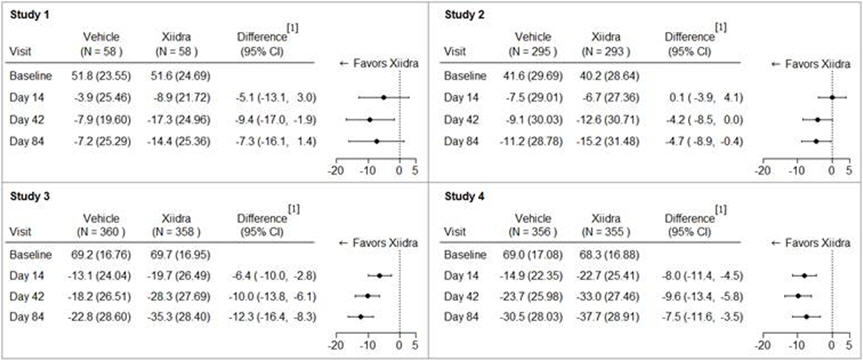
Eye dryness score was rated by patients using a visual analogue scale (0 = no discomfort, 100 = maximal discomfort) at each study visit. The average baseline EDS was between 40 and 70. A larger reduction in EDS favoring Xiidra was observed in all studies at Day 42 and Day 84 (see Figure 1).
Figure 1: Mean Change (SD) From Baseline and Treatment Difference (Xiidra – Vehicle) in Eye Dryness Score in 12-Week Studies in Patients With Dry Eye Disease
[1] Based on analysis of covariance (ANCOVA) model adjusted for baseline value in Study 1, and ANCOVA model adjusted for baseline value and randomization stratification factors in Studies 2-4. All randomized and treated patients were included in the analysis and missing data were imputed using last-available data. In Study 1, one Xiidra-treated subject who did not have a baseline value was excluded from analysis.
Effects on Signs of Dry Eye Disease
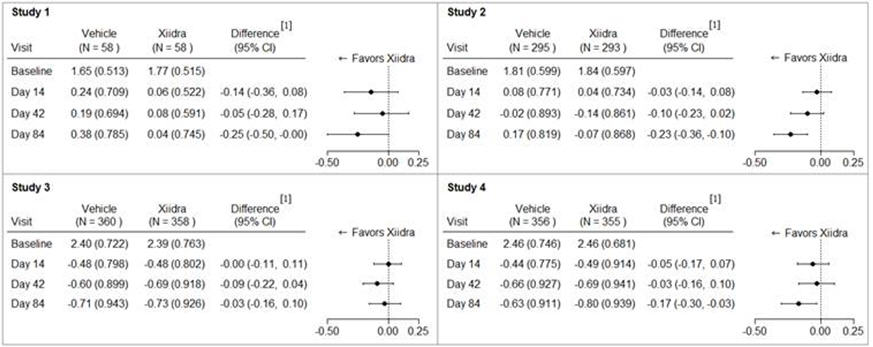
Inferior fluorescein corneal staining score (ICSS) (0 = no staining, 1 = few/rare punctate lesions, 2 = discrete and countable lesions, 3 = lesions too numerous to count but not coalescent, 4 = coalescent) was recorded at each study visit. The average baseline ICSS was approximately 1.8 in Studies 1 and 2, and 2.4 in Studies 3 and 4. At Day 84, a larger reduction in ICSS favoring Xiidra was observed in three of the four studies (see Figure 2).
Figure 2: Mean Change (SD) From Baseline and Treatment Difference (Xiidra – Vehicle) in Inferior Corneal Staining Score in 12-Week Studies in Patients With Dry Eye Disease
[1] Based on ANCOVA model adjusted for baseline value in Study 1, and ANCOVA model adjusted for baseline value and randomization stratification factors in Studies 2-4. All randomized and treated patients were included in the analysis and missing data were imputed using last-available data. In Study 2, one vehicle-treated subject who did not have a study eye designated was excluded from analysis.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Xiidra (lifitegrast ophthalmic solution) 5% (50 mg/mL) is supplied in a foil pouch containing 5 low-density polyethylene 0.2 mL single-use containers.
Carton of 60 single-use containers NDC: 0078-0911-12
Storage:
Store at 20°C to 25°C (68°F to 77°F). Store single-use containers in the original foil pouch.
-
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Handling the Single-use Container
Advise patients not to touch the tip of the single-use container to their eye or to any surface, in order to avoid eye injury or contamination of the solution.
Use With Contact Lenses
Advise patients that contact lenses should be removed prior to administration of Xiidra and can be reinserted 15 minutes after administration [see Dosage and Administration (2)].
Administration
Advise patients that the solution from one single-use container is to be used immediately after opening. It can be used to dose both eyes. The single-use container, including any remaining contents should be discarded immediately after administration [see Dosage and Administration (2)].
Storage Information
Instruct patients to store single-use containers in the original foil pouch until ready to use [see How Supplied/Storage and Handling (16)].
Distributed by:
Novartis Pharmaceuticals Corporation
One Health Plaza
East Hanover, NJ 07936T2020-87
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: June 2020
PATIENT INFORMATION
XIIDRA ®(ZYE-druh)
(lifitegrast ophthalmic solution) 5%
for topical ophthalmic useWhat is Xiidra?
Xiidra is a prescription eye drop solution used to treat the signs and symptoms of dry eye disease (DED). It is not known if Xiidra is safe and effective in children under 17 years of age.Do not use Xiidra:
- If you are allergic to lifitegrast or any of the other ingredients in Xiidra, see “What are the ingredients in Xiidra?”
Before you use Xiidra, tell your doctor if you:
- are using any other eye drops
- wear contact lenses
- are pregnant or plan to become pregnant. It is not known if Xiidra will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Xiidra passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use Xiidra.
How should I use Xiidra?
See the complete Instructions for Use at the end of this Patient Information leaflet for detailed instructions about the right way to use Xiidra.- Use Xiidra as your doctor tells you.
- Use one drop of Xiidra in each eye, two times each day, about 12 hours apart.
- Use Xiidra right away after opening. Throw away the single-use container and any unused solution after you have applied the dose to both eyes. Do not save any unused Xiidra for later.
What are the possible side effects of Xiidra?
The most common side effects of Xiidra include eye irritation, discomfort, or blurred vision when the drops are applied to the eyes, and an unusual taste sensation (dysgeusia).
Seek medical care immediately if you get any symptoms of wheezing, difficulty breathing, or swollen tongue.
These are not all the possible side effects of Xiidra.
Tell your doctor if you have any side effects that bother you. You may report side effects to FDA at 1-800-FDA-1088.How should I store Xiidra?
- Store Xiidra at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Xiidra in the original foil pouch to protect it from light.
- Do not open the Xiidra foil pouch until you are ready to use the eye drops.
- Return unused single-use containers to their original foil pouch to protect from excessive light exposure.
Keep Xiidra and all medicines out of the reach of children.
General information about the safe and effective use of Xiidra.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or doctor for information about Xiidra that is written for health professionals. Do not use Xiidra for a condition for which it was not prescribed. Do not give Xiidra to other people, even if they have the same symptoms you have. It may harm them.What are the ingredients in Xiidra?
Active ingredient: lifitegrast
Inactive ingredients: sodium chloride, sodium phosphate dibasic anhydrous, sodium thiosulfate pentahydrate, and water for injection. Sodium hydroxide and/or hydrochloric acid (to adjust pH).Distributed by: Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936
For more information, go to www.Xiidra.com or call 1-888-NOW-NOVA.T2020-88
-
INSTRUCTIONS FOR USE
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: June 2020
INSTRUCTIONS FOR USE
XIIDRA ®[ZYE-druh]
(lifitegrast ophthalmic solution) 5%
for topical ophthalmic use- Read this Instructions for Use before you start using Xiidra and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or your treatment.
Important Information You Need to Know Before Using Xiidra:
- Xiidra is for use in the eye.
- Wash your hands before each use to make sure you do not infect your eyes while using Xiidra.
- If you wear contact lenses, remove them before using Xiidra.
- Xiidra single-use containers are packaged in a foil pouch. Do not remove from the foil pouch until you are ready to use Xiidra.
- Do not let the tip of the Xiidra single-use container touch your eye or any other surfaces.
- Use one drop of Xiidra in each eye two times each day (one drop in the morning and one drop in the evening, approximately 12 hours apart). Each single-use container of Xiidra will give you enough medicine to treat both of your eyes, one time. There is some extra Xiidra in each single-use container in case you miss getting a drop into your eye. After you have applied the drops, throw away the single-use container and any unused Xiidra. Do not save any unused Xiidra.
Follow Steps 1 to 9 each time you use Xiidra.
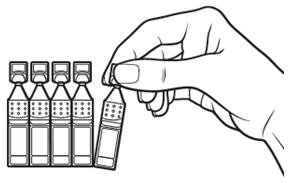
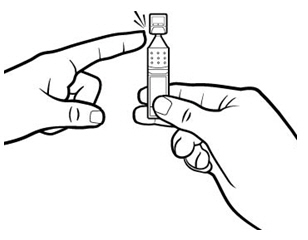
Step 1.Take a foil pouch out of the Xiidra box. Open the pouch and remove the strip of single-use containers (see Figure A).
- Pull off one single-use container from the strip (see Figure B).
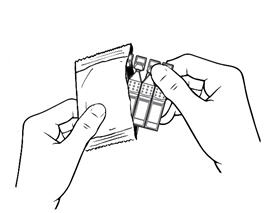
Step 2.Put the remaining strip of single-use containers back in the pouch (see Figure C).
- Fold the edge to close the pouch (see Figure D).
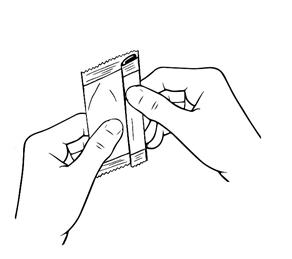
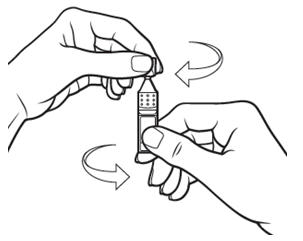
Step 3.Hold the Xiidra container upright (see Figure E).
- Tap the top of the container until all of the solution is in the bottom part of the container (see Figure F).
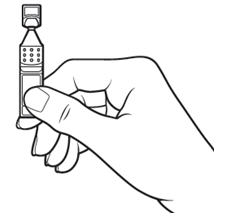
Step 4.Open the Xiidra single-use container by twisting off the tab. Make sure that the tip of the single-use container does not touch anything, to avoid contamination (see Figure G).
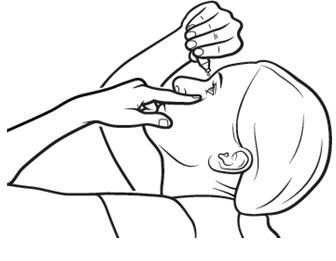
Step 5.Tilt your head backwards. If you are not able to tilt your head, lie down.
Step 6.Gently pull your lower eyelid downwards and look up.
Step 7.Place the tip of the Xiidra single-use container close to your eye, but be careful not to touch your eye with it.
Step 8.Gently squeeze the single-use container and let one drop of Xiidra fall into the space between your lower eyelid and your eye. If a drop misses your eye, try again (see Figure H).
Step 9. Repeat Steps 5 to 8for your other eye. There is enough Xiidra in one single-use container for both eyes.
- Once you have applied a drop to both eyes, throw away the opened single-use container with any remaining solution.
- If you use contact lenses, wait for at least 15 minutes before placing them back in your eyes.
Distributed by: Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936
T2020-89
-
PRINCIPAL DISPLAY PANEL
NDC: 0078-0911-12
Rx Only
60 Single-Use
Containers:
12 pouches x 5 single-use
containers (0.2 mL each)xiidra ®
(lifitegrast
ophthalmic solution) 5%NOVARTIS
-
INGREDIENTS AND APPEARANCE
XIIDRA
lifitegrast solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0911 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIFITEGRAST (UNII: 038E5L962W) (LIFITEGRAST - UNII:038E5L962W) LIFITEGRAST 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM THIOSULFATE (UNII: HX1032V43M) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0911-12 12 in 1 CARTON 06/04/2020 06/01/2027 1 NDC: 0078-0911-05 5 in 1 POUCH 1 0.2 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 0078-0911-94 4 in 1 CARTON 06/04/2020 02/01/2027 2 NDC: 0078-0911-95 5 in 1 POUCH 2 0.2 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208073 07/11/2016 Labeler - Novartis Pharmaceuticals Corporation (002147023) Establishment Name Address ID/FEI Business Operations The Ritedose Corporation 837769546 manufacture(0078-0911)
Trademark Results [Xiidra]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XIIDRA 87319276 5271076 Live/Registered |
SARcode Bioscience Inc. 2017-01-31 |
 XIIDRA 86056637 5050074 Live/Registered |
SARcode Bioscience Inc. 2013-09-05 |
 XIIDRA 79293316 not registered Live/Pending |
NOVARTIS AG 2020-06-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.