DEXTROSE- dextrose monohydrate injection, solution
Dextrose by
Drug Labeling and Warnings
Dextrose by is a Prescription medication manufactured, distributed, or labeled by Hospira, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Dextrose Injection, USP solution is sterile and nonpyrogenic. It is a parenteral solution containing dextrose in water for injection intended for intravenous administration.
Each 100 mL of 5% Dextrose Injection, USP, contains dextrose, hydrous 5 g in water for injection. The caloric value is 170 kcal/L. The osmolarity is 252 mOsmol/L (calc.), which is slightly hypotonic.
Each 100 mL of 10% Dextrose Injection, USP, contains dextrose, hydrous 10 g in water for injection. The caloric value is 340 kcal/L. The osmolarity is 505 mOsmol/L (calc.), which is hypertonic.
The solution pH for both concentrations is 4.3 (3.2 to 6.5).
The solutions contain no bacteriostat, antimicrobial agent or added buffer and each is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
The solutions are parenteral fluid and nutrient replenishers.
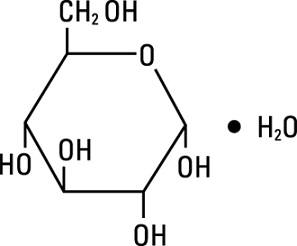
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a clear multilayer polyolefin plastic film. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
-
CLINICAL PHARMACOLOGY
When administered intravenously, these solutions provide a source of water and carbohydrate.
Isotonic and hypertonic concentrations of dextrose are suitable for parenteral maintenance of water requirements when salt is not needed or should be avoided.
Solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Water is an essential constituent of all body tissues and accounts for approximately
70% of total body weight. Average normal adult daily requirements range from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Excessive administration of potassium-free solutions may result in significant hypokalemia.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
-
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Studies with Dextrose Injection, USP have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy: Teratogenic effects
Pregnancy Category C. Animal reproduction studies have not been conducted with dextrose.
It is also not known whether dextrose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose should be given to a pregnant woman only if clearly needed.Nursing Mothers: Caution should be exercised when Dextrose Injection, USP is administered to a nursing mother.
Pediatric Use: The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants the volume of fluid may affect fluid and electrolyte balance.
Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolarity and possible intracerebral hemorrhage.
Geriatric Use: An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
In the event of overhydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.
-
DOSAGE AND ADMINISTRATION
The dose is dependent upon the age, weight and clinical condition of the patient.
As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
Drug Interactions
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
-
INSTRUCTIONS FOR USE
Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired. If supplemental medication is desired, follow directions below before preparing for administration.
To Add Medication
(Use aseptic technique)
- 1. Remove blue cap from BLU-MED™ sterile medication additive port at bottom of container.
- 2. With a needle of appropriate length, puncture resealable additive port and inject. Withdraw needle after injecting medication.
- 3. Mix container contents thoroughly.
- 4. The additive port may be protected by an appropriate cover.
Preparation for Administration
(Use aseptic technique)
NOTE: See appropriate I.V. administration set Instructions for Use.
- 1. Close flow control clamp of administration set.
- 2. Remove cap from sterile administration set port at bottom of container.
- 3. Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
- 4. Suspend container.
- 5. Squeeze and release drip chamber to establish proper fluid level in chamber.
- 6. Open clamp. Eliminate air from remainder of set.
- 7. Attach set to patient access device.
- 8. Begin infusion.
WARNING: Do not use flexible container in series connections.
-
HOW SUPPLIED
Dextrose Injection, USP is supplied in single-dose flexible plastic containers as follows:
NDC
Product Name
Fill Volume/Container size (mL)
0409-7922-21
5% Dextrose Injection, USP
150/250
0409-7922-25
5% Dextrose Injection, USP
250/250
0409-7922-30
5% Dextrose Injection, USP
500/500
0409-7922-48
5% Dextrose Injection, USP
1000/1000
0409-7923-03
5% Dextrose Injection, USP
25/50
0409-7923-06
5% Dextrose Injection, USP
50/50
0409-7923-11
5% Dextrose Injection, USP
100/100
0409-7930-25
10% Dextrose Injection, USP
250/250
0409-7930-30
10% Dextrose Injection, USP
500/500
0409-7930-48
10% Dextrose Injection, USP
1000/1000
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Revised: June, 2009
Printed in USA EN-2166

Hospira, Inc., Lake Forest, IL 60045 USA -
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label
250 mL
VisIV™ ContainerNCD 0409-7922-25
5% DEXTROSE
Injection, USPEACH 100 mL CONTAINS DEXTROSE,
HYDROUS 5 g IN WATER FOR INJECTION.252 mOsmol/LITER (CALC.)
pH 4.3 (3.2 to 6.5)
DEXTROSE SOLUTIONS WITHOUT SALTS
SHOULD NOT BE USED IN BLOOD
TRANSFUSIONS BECAUSE OF POSSIBLE
ROULEAU FORMATION.ADDITIVES MAY BE INCOMPATIBLE.
CONSULT WITH PHARMACIST, IF
AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE,
MIX THOROUGHLY AND DO NOT
STORE. SINGLE-DOSE CONTAINER. FOR
INTRAVENOUS USE. USUAL DOSAGE:
SEE INSERT. STERILE, NONPYROGENIC.
STORE AT 20 TO 25°C (68 TO 77°F).
[SEE USP CONTROLLED ROOM
TEMPERATURE.] PROTECT FROM
FREEZING. SEE INSERT. USE ONLY IF
SOLUTION IS CLEAR AND CONTAINER IS
UNDAMAGED. MUST NOT BE USED IN
SERIES CONNECTIONS. PVC-FREE,
DEHP-FREE. VisIV IS A TRADEMARK OF
HOSPIRA. DO NOT REMOVE CAPS UNTIL
READY FOR USE. IF LEAKS ARE FOUND,
DISCARD SOLUTION AS STERILITY MAY
BE IMPAIRED.Rx ONLY
5
PPIM-1695 (7/08)
PRINTED IN USA
HOSPIRA, INC.
LAKE FOREST, IL 60045 USAHospira
MED
SET -
PRINCIPAL DISPLAY PANEL - Bag Overwrap - 7922
TO OPEN TEAR AT NOTCH
2
HDPEDO NOT REMOVE FROM OVERWRAP UNTIL READY FOR USE. AFTER REMOVING
THE OVERWRAP, CHECK FOR MINUTE LEAKS BY SQUEEZING CONTAINER FIRMLY.
IF LEAKS ARE FOUND, DISCARD SOLUTION AS STERILITY MAY BE IMPAIRED.
RECOMMENDED STORAGE: ROOM TEMPERATURE (25°C). AVOID EXCESSIVE
HEAT. PROTECT FROM FREEZING. SEE INSERT.
98-4321-R14-3/98
-
PRINCIPAL DISPLAY PANEL - Bag Overwrap - 50 mL
TO OPEN – TEAR AT NOTCH
One Unit5% DEXTROSE
Injection, USP
50 mLEach 100 mL contains dextrose, hydrous 5 g.
252 mOsmol/liter (CALC.).
pH 4.3 (3.2 to 6.5).Dextrose solutions without salts should not be used in blood transfusions because
of possible rouleau formation.Additives may be incompatible. Consult with pharmacist, if available. When
introducing additives, use aseptic technique, mix thoroughly and do not store.Single-dose container. For I.V. use. Usual dosage: See insert. Sterile,
nonpyrogenic. Use only if solution is clear. After removing the overwrap, check for
minute leaks by squeezing container firmly. If leaks are found, discard unit as
sterility may be impaired. Must not be used in series connections.
The overwrap is a moisture barrier. Do not remove unit from overwrap until ready
for use. Use unit promptly when pouch is opened. Store at 20 to 25°C (68 to 77°F).
[See USP Controlled Room Temperature.] Protect from freezing. See insert.Rx only
Printed in USA
F WR-0288 (4/08)Hospira, Inc., Lake Forest, IL 60045 USA
Hospira
-
PRINCIPAL DISPLAY PANEL - 100 mL Bag Label
100 mL
VisIV™ ContainerNDC: 0409-7923-11
5% DEXTROSE
INJECTION, USPEACH 100 mL CONTAINS DEXTROSE, HYDROUS 5 g.
252 mOsmol/LITER (CALC.).
pH 4.3 (3.2 to 6.5).DEXTROSE SOLUTIONS WITHOUT SALTS SHOULD NOT BE USED IN BLOOD
TRANSFUSIONS BECAUSE OF POSSIBLE ROULEAU FORMATION. ADDITIVES
MAY BE INCOMPATIBLE. SINGLE-DOSE CONTAINER. FOR
INTRAVENOUS USE. USUAL DOSAGE: SEE INSERT. STERILE,
NONPYROGENIC. STORE AT 20 TO 25°C (68 to 77°F). [SEE
USP CONTROLLED ROOM TEMPERATURE.] PROTECT FROM
FREEZING. SEE INSERT. USE ONLY IF SOLUTION IS CLEAR
AND CONTAINER IS UNDAMAGED. MUST NOT BE USED IN
SERIES CONNECTIONS. PVC-FREE, DEHP-FREE. DO NOT
REMOVE CAPS UNTIL READY FOR USE. IF LEAKS ARE
FOUND, DISCARD SOLUTION AS STERILITY MAY BE
IMPAIRED.Rx ONLY
5
PPPRINTED IN USA
IM-1734 (9/08)
HOSPIRA, INC., LAKE FOREST, IL 60045 USA
HospiraMED
SET -
INGREDIENTS AND APPEARANCE
DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0409-7922 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-7922-25 24 in 1 CASE 04/16/2009 1 250 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0409-7922-30 24 in 1 CASE 07/26/2006 05/01/2013 2 500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 0409-7922-48 12 in 1 CASE 10/31/2006 11/01/2013 3 1000 mL in 1 BAG; Type 0: Not a Combination Product 4 NDC: 0409-7922-21 48 in 1 CASE 09/09/2005 09/10/2005 4 150 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016367 09/09/2005 DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0409-7923 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-7923-06 60 in 1 CASE 11/24/2009 1 50 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0409-7923-11 60 in 1 CASE 11/24/2009 2 100 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 0409-7923-03 60 in 1 CASE 09/09/2005 09/10/2005 3 25 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016367 09/09/2005 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-7922, 0409-7923)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.