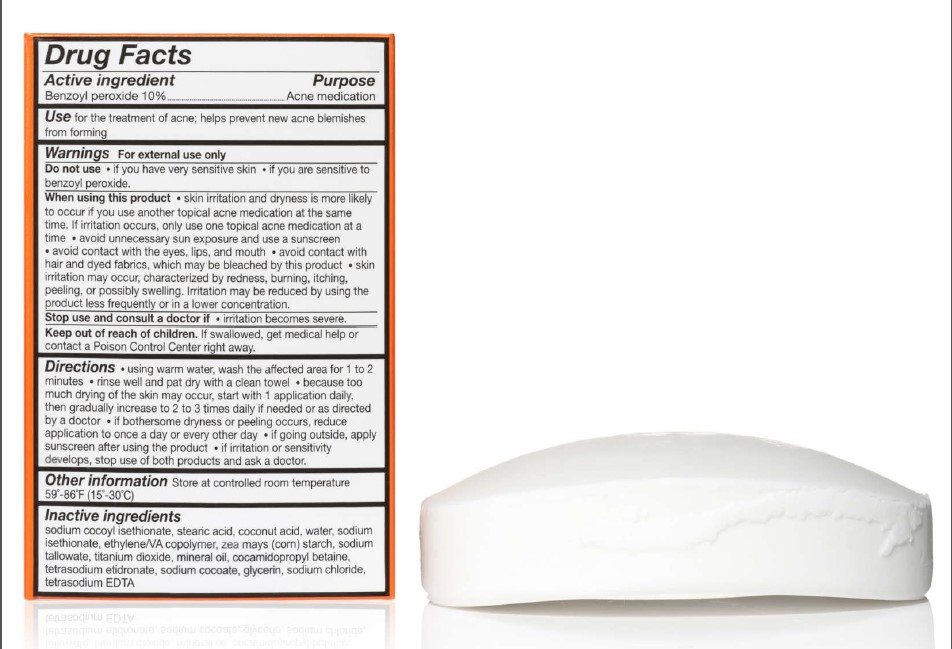
111 MEDCO BENZOYL PEROXIDE- benzoyl peroxide soap
111 Medco Benzoyl Peroxide by
Drug Labeling and Warnings
111 Medco Benzoyl Peroxide by is a Otc medication manufactured, distributed, or labeled by 111 Medco. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
DOSAGE & ADMINISTRATION
Directions
- using warm water, wash the affected area for 1 to 2 minutes
- rinse well and pat dry with a clean towel
- because too much drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using the product
- If irritation or sensitivity develops, stop use of both products and ask a doctor.
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
sodium cocoyl isethionate, stearic acid, coconut acid, water, sodium isethionate, ethylene/VA copolymer, zea mays (corn) starch, sodium tallowate, titanium dioxide, mineral oil, cocamidopropyl betaine, tetrasodium etidronate, sodium cocoate, glycerin, sodium chloride, tetrasodium EDTA
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
111 MEDCO BENZOYL PEROXIDE
benzoyl peroxide soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72811-137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM TALLOWATE, BEEF (UNII: 07RIK6QMEW) COCONUT ACID (UNII: 40U37V505D) WATER (UNII: 059QF0KO0R) SODIUM ISETHIONATE (UNII: 3R36J71C17) ETHYLENE-VINYL ACETATE COPOLYMER (15% VINYL ACETATE) (UNII: V9BQI51YUL) STARCH, CORN (UNII: O8232NY3SJ) SODIUM COCOATE (UNII: R1TQH25F4I) GLYCERIN (UNII: PDC6A3C0OX) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MINERAL OIL (UNII: T5L8T28FGP) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE SODIUM (UNII: MP1J8420LU) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72811-137-11 113 g in 1 CARTON; Type 0: Not a Combination Product 04/02/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 04/02/2019 Labeler - 111 Medco (065115643)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.