Lumin Broad Spectrum Moisturizer with SPF 30 PA UVA/UVB Protection Sunscreen
Lumin Broad Spectrum Moisturizer with SPF 30 PA UVA/UVB Protection Sunscreen by
Drug Labeling and Warnings
Lumin Broad Spectrum Moisturizer with SPF 30 PA UVA/UVB Protection Sunscreen by is a Otc medication manufactured, distributed, or labeled by Pangaea Holdings Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
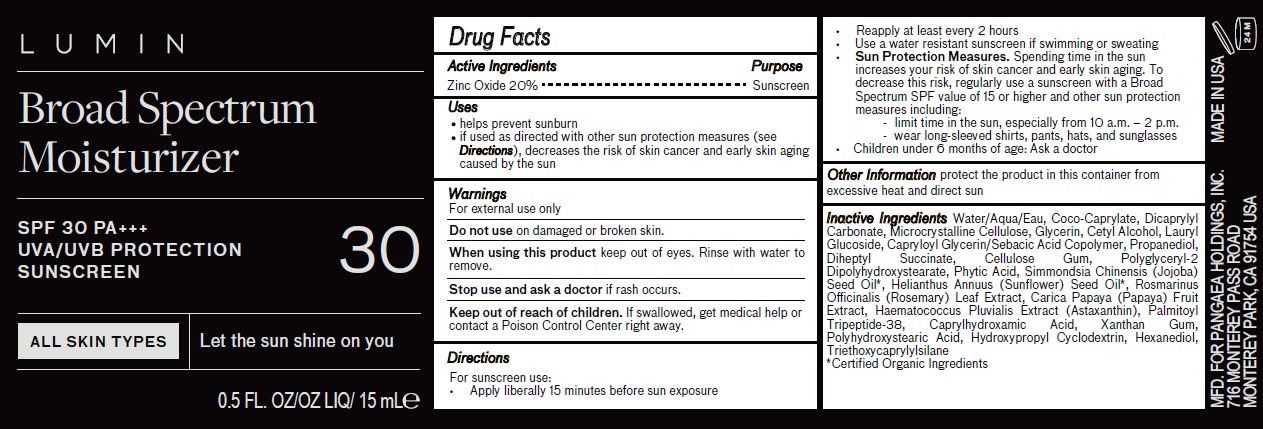
LUMIN BROAD SPECTRUM MOISTURIZER WITH SPF 30 PA UVA/UVB PROTECTION SUNSCREEN- zinc oxide cream
Pangaea Holdings Inc.
----------
Lumin Broad Spectrum Moisturizer with SPF 30 PA UVA/UVB Protection Sunscreen
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
Apply liberally 15 minutes before sun exposure
Reapply at least every 2 hours
Use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Children under 6 months ofOther Information age: Ask a doctor
Inactive Ingredients
Water/Aqua/Eau, Coco-Caprylate, Dicaprylyl Carbonate, Microcrystalline Cellulose, Glycerin, Cetyl Alcohol, Lauryl Glucoside, Capryloyl Glycerin/Sebacic Acid Copolymer, Propanediol, Diheptyl Succinate, Cellulose Gum, Polyglyceryl-2 Dipolyhydroxystearate, Phytic Acid, Simmondsia Chinensis (Jojoba) Seed Oil*, Helianthus Annuus (Sunflower) Seed Oil*, Rosmarinus Officinalis (Rosemary) Leaf Extract, Carica Papaya (Papaya) Fruit Extract, Haematococcus Pluvialis Extract (Astaxanthin), Palmitoyl Tripeptide-38, Caprylhydroxamic Acid, Xanthan Gum, Polyhydroxystearic Acid, Hydroxypropyl Cyclodextrin, Hexanediol, Triethoxycaprylylsilane
*Certified Organic Ingredients
| LUMIN BROAD SPECTRUM MOISTURIZER WITH SPF 30 PA UVA/UVB PROTECTION SUNSCREEN
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Pangaea Holdings Inc. (081181313) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.