SUL-Q-NOX- sulfaquinoxaline solution
Sul-Q-Nox by
Drug Labeling and Warnings
Sul-Q-Nox by is a Animal medication manufactured, distributed, or labeled by Huvepharma, Inc, Huvepharma EOOD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
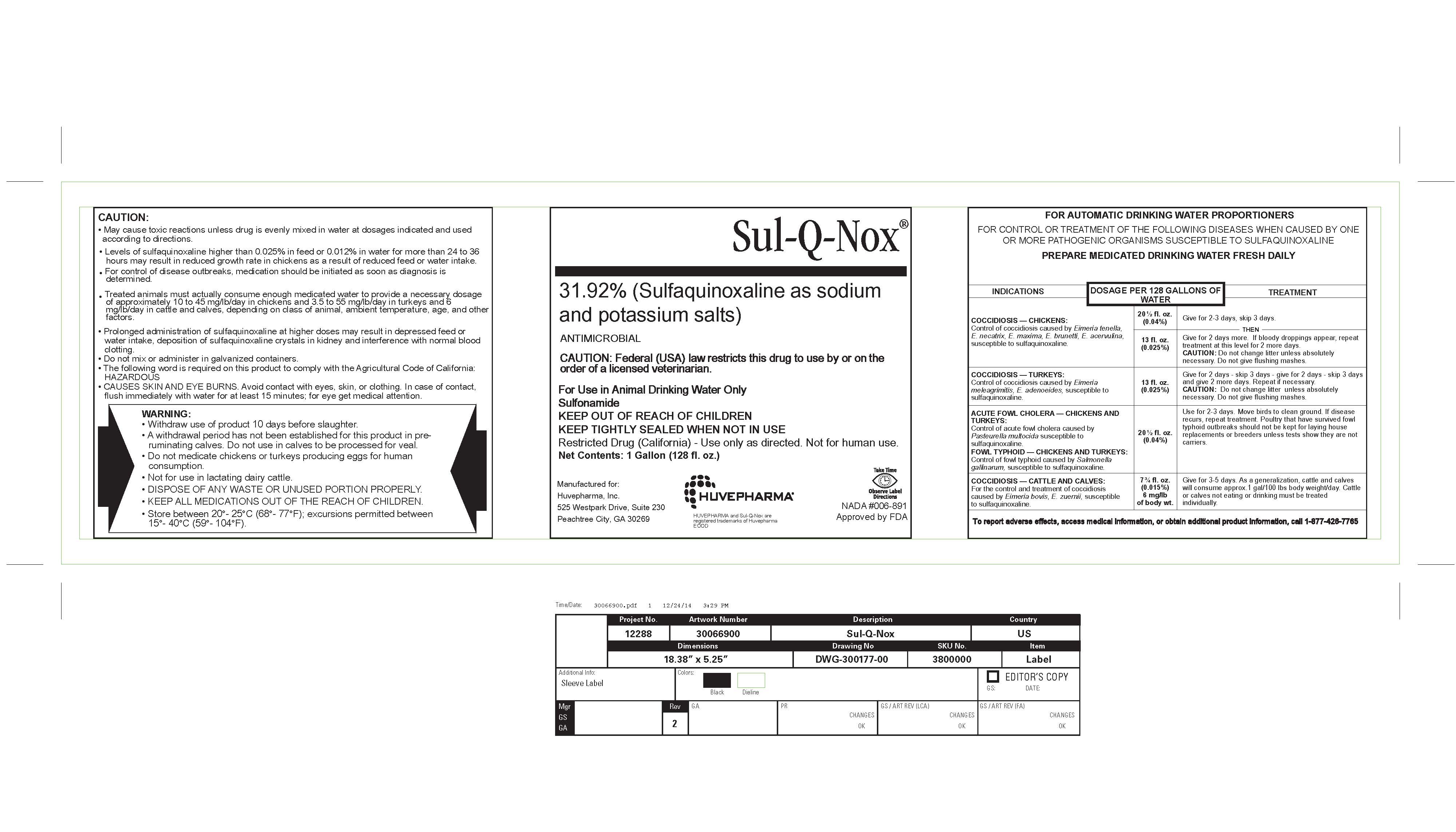
Front Panel
Sul-Q-Nox®
31.92% (Sulfaquinoxaline as sodium
and potassium salts)ANTIMICROBIAL
Net Contents: 26 Fluid Ounces (.76 Liters)Manufactured for:
Huvepharma, Inc.
525 Westpark Drive, Suite 230
Peachtree City, GA 30269HUVEPHARMA and Sul-Q-Nox are
registered trademarks of Huvepharma
EOODNADA #006-891
Approved by FDA - Caution:
-
CAUTION:
May cause toxic reactions unless drug is even mixed in water at dosages indicated and use according
to directions.
Levels of sulfaquinoxaline higher than 0.025% in feed or 0.012% in water for more than 24 to 36 hours
may result in reduced growth rate in chickens as a result of reduced feed or water intake.
For control of disease outbreaks, medication should be initiated as soon as diagnosis is determined.
Treated animals must actually consume enough medicated water to provide a necessary dosage of
approximately 10 to 45 mg/lb/day in chickens and 3.5 to 55 mg/lb/day in turkeys and 6 mg/lb/day in
cattle and calves, depending on class of animal, ambient temperature, age, and other factors.
Prolonged administration of sulfaquinoxaline at higher doses may result in depressed feed or water
intake, deposition of sulfaquinoxaline crystals in kidney and interference with normal blood clotting.
Do not mix or administer in galvanized containers.
The follwing word is required on this product to comply with Agricultural Code of California:
HAZARDOUS
CAUSES SKIN AND EYE BURNS. Avoid contact with eyes, skin, or clothing. In case of contact, flush
immediately with water for at least 15 minutes; for eye get medical attention. -
WARNING:
Withdraw use of product 10 days before slaughter.
A withdrawal period has not been established for this product in preruminating
calves. Do not use in calves to be processed for veal.
Do not medicate chickens or turkeys producing eggs for human
consumption.
Not for use in lactating dairy cattle.
DISPOSE OF ANY WASTE OR UNUSED PORTION PROPERLY.
KEEP ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store between 20°- 25°C (68°- 77°F); excursions permitted between
15°- 40°C (59°- 104°F). -
INDICATIONS & USAGE
FOR AUTOMATIC DRINKING WATER PROPORTIONERS
FOR CONTROL OR TREATMENT OF THE FOLLOWING DISEASES WHEN CAUSED BY ONE
OR MORE PATHOGENIC ORGANISMS SUSCEPTIBLE TO SULFAQUINOXALINE
PREPARE MEDICATED DRINKING WATER FRESH DAILYINDICATIONS DOSAGE PER 128 GALLONS OF
WATERTREATMENT COCCIDIOSIS — CHICKENS:
Control of coccidiosis caused by Eimeria tenella,
E. necatrix, E. maxima, E. brunetti, E. acervulina,
susceptible to sulfaquinoxaline.20½ fl. oz.
(0.04%)Give for 2-3 days, skip 3 days.
Then
13 fl. oz.
(0.025%)Give for 2 days more. If bloody droppings appear, repeat
treatment at this level for 2 more days.
CAUTION: Do not change litter unless absolutely
necessary. Do not give flushing mashes.COCCIDIOSIS — TURKEYS:
Control of coccidiosis caused by Eimeria
meleagrimitis, E. adenoeides, susceptible to
sulfaquinoxaline.13 fl. oz.
(0.025%)Give for 2 days - skip 3 days - give for 2 days - skip 3 days
and give 2 more days. Repeat if necessary.
CAUTION: Do not change litter unless absolutely
necessary. Do not give flushing mashes.ACUTE FOWL CHOLERA — CHICKENS AND
TURKEYS:
Control of acute fowl cholera caused by
Pasteurella multocida susceptible to
sulfaquinoxaline.
FOWL TYPHOID — CHICKENS AND TURKEYS:
Control of fowl typhoid caused by Salmonella
gallinarum, susceptible to sulfaquinoxaline.20½ fl. oz.
(0.04%)Use for 2-3 days. Move birds to clean ground. If disease
recurs, repeat treatment. Poultry that have survived fowl
typhoid outbreaks should not be kept for laying house
replacements or breeders unless tests show they are not
carriers.COCCIDIOSIS — CATTLE AND CALVES:
For the control and treatment of coccidiosis
caused by Eimeria bovis, E. zuernii, susceptible
to sulfaquinoxaline.7¾ fl. oz.
(0.015%)
6 mg/lb
of body wt.Give for 3-5 days. As a generalization, cattle and calves
will consume approx.1 gal/100 lbs body weight/day. Cattle
or calves not eating or drinking must be treatedindividually.
To report adverse effects, access medical information, or obtain additional product information, call 1-877-426-7765 - Take Time Image
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUL-Q-NOX
sulfaquinoxaline solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 23243-6771 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFAQUINOXALINE (UNII: WNW8115TM9) (SULFAQUINOXALINE - UNII:WNW8115TM9) SULFAQUINOXALINE .3192 g in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 23243-6771-2 4 in 1 BOX 1 3785 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA006891 07/09/2019 Labeler - Huvepharma, Inc (619153559) Registrant - Huvepharma EOOD (552691651) Establishment Name Address ID/FEI Business Operations Huvepharma, Inc 080346321 analysis, label, manufacture, pack
Trademark Results [Sul-Q-Nox]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUL-Q-NOX 86244066 not registered Dead/Abandoned |
Zoetis LLC 2014-04-07 |
 SUL-Q-NOX 86051931 4909198 Live/Registered |
ZOETIS LLC 2013-08-29 |
 SUL-Q-NOX 78715966 3212506 Dead/Cancelled |
ZOETIS PRODUCTS LLC 2005-09-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.