COSACTHEN- cosyntropin injection
CosACTHen by
Drug Labeling and Warnings
CosACTHen by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION
Cosyntropin consists of the first 24 amino acids in naturally occurring adrenocorticotropic hormone (ACTH). The sequence of amino acids in cosyntropin is as follows:
Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg-Arg-Pro-Val- Lys-Val-Tyr-Pro
Molecular formula: C136H210N40O31S
Cosacthen® (cosyntropin injection) is a clear, aqueous solution. Each milliliter contains 0.25 mg cosyntropin, 1 mg glacial acetic acid, 0.82 mg sodium acetate trihydrate, 8.1 mg sodium chloride and water for injection (to 100%).
- INDICATION
-
DOSAGE AND ADMINISTRATION
The dose is 0.25 mg (1 mL) per dog weighing 10–110 lbs (4.5–50 kg), administered by intravenous or intramuscular injection, with the purpose of performing the adrenocorticotropic hormone (ACTH) stimulation test. Discard unused portion. Cosacthen is provided in a single use vial and does not contain a preservative.
Collect the first blood sample to measure the baseline cortisol concentration immediately prior to administering Cosacthen. Collect the second blood sample 1 hour after administration of Cosacthen to measure the dog’s cortisol concentration in response to Cosacthen.
- CONTRAINDICATIONS
-
WARNINGSUSER SAFETY WARNINGS
Not for use in humans. Keep this and all drugs out of reach of children.
People with known hypersensitivity to cosyntropin or ACTH should avoid contact with the product.
Pregnant women and breastfeeding women should take care to avoid accidental self-injection.
If any anaphylactic or allergic symptoms (skin reactions, dizziness, nausea, vomiting, urticaria, pruritus, flushing, malaise, dyspnea, angioneurotic edema) develop following exposure to Cosacthen, medical advice must be sought immediately. In case of accidental self-injection, seek medical advice immediately and show the package insert or label to the physician.
If skin contact occurs, wash affected area with soap and water.
- PRECAUTIONS
-
ADVERSE REACTIONS
In the field study using a non-final formulation cosyntropin injection, which included 119 dogs with suspected hypoadrenocorticism or hyperadrenocorticism, two dogs vomited within 8 hours after cosyntropin injection administration, and one dog developed a hematoma at the injection site after IV administration. Clinical pathology abnormalities were consistent with pre-existing hypoadrenocorticism and hyperadrenocorticism.
Foreign Market Experience: The following adverse events were reported voluntarily during post-approval use of Cosacthen in dogs in foreign markets: lethargy, anxiety, muscle tremor/weakness, abdominal pain, anorexia, diarrhea, injection site pain/bruising, lameness, and hypersensitivity reactions.
CONTACT INFORMATION
To report suspected adverse drug experiences, or for technical assistance, contact Dechra Veterinary Products at (866) 933-2472.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae
-
CLINICAL PHARMACOLOGY
Cosyntropin, like ACTH, binds specific receptors in the plasma membrane of adrenal cortical cells in the zona fasciculata. The hormone-receptor complex activates adenylate cyclase, which stimulates the production of cyclic AMP. This leads to the conversion of cholesterol to pregnenolone, and thus to the production of various glucocorticoids via their respective synthetic pathways.
-
EFFECTIVENESS
Cosacthen was evaluated in a laboratory effectiveness study in 16 healthy, 9- to 56-month-old beagle dogs. Eight dogs were administered Cosacthen intramuscularly (IM) at a dose of 0.005 mg/kg. Eight control dogs were administered saline. All dogs in the Cosacthen group had a positive response (defined as a change from a pre-dose serum cortisol concentration of 0.5–6.5 μg/dL to a post-dose serum cortisol concentration greater than 6.5 μg/dL) at 30, 60, and 90 minutes after administration. No dogs in the control group had a positive response.
The mean serum cortisol concentration 60 minutes after Cosacthen or saline administration was 12.9 μg/dL (range 10.6–14.6 μg/dL) and 2.3 μg/dL (range 0.8–3.4 μg/dL), respectively.
A non-final formulation of cosyntropin injection was evaluated in a randomized, unmasked multi-site field study to evaluate effectiveness, diagnostic performance, and safety. One hundred nineteen dogs suspected of having hypoadrenocorticism (21) or hyperadrenocorticism (98) were randomized to two treatment groups and administered cosyntropin injection at 0.25 mg/dog once by IM or IV injection. Blood samples were collected prior to dosing to assess baseline serum cortisol concentrations and again 60 minutes after cosyntropin injection administration.
Diagnostic performance was evaluated in 107 dogs and safety was evaluated in 119 dogs. The true and false negative and positive results are summarized in Table 1. The accuracy, negative and positive predictive values, sensitivity, and specificity results are summarized in Table 2.
Table 1. True and False Negative and Positive Results for Non-Final Formulation Cosyntropin Injection
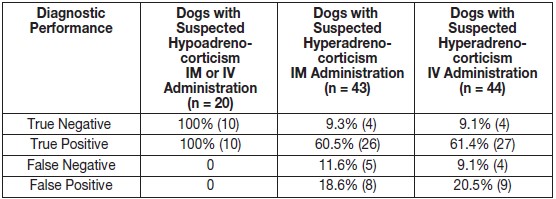
Table 2. Accuracy, Negative and Positive Predictive Value, Sensitivity, and Specificity of Non-Final Formulation Cosyntropin Injection
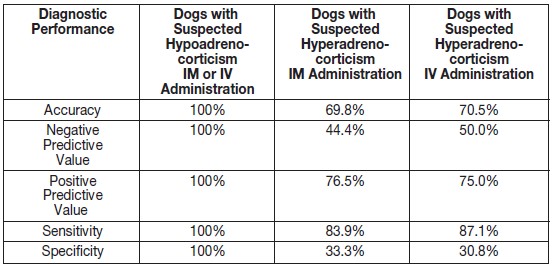
Two dogs vomited within 8 hours after cosyntropin injection administration. One dog developed a hematoma following IV administration of cosyntropin injection.
-
TARGET ANIMAL SAFETY
A non-final formulation of cosyntropin injection was administered to 32 healthy, 5- to 6-month-old beagle dogs as 3 weekly IV or IM injections. Twenty IV-dosed dogs were administered multiples of 0X (4), 1X (4), 3X (4), or 5X (8) and 12 IM-dosed dogs were administered multiples of 0X (4), 1X (4), or 2X (4) the maximum exposure dose of 0.056 mg/kg. Control dogs were administered saline.
All dogs completed the study. Clinical signs related to cosyntropin injection administration included transient salivation during and/or immediately after dosing in six of eight dogs in the 5X, IV-dosed group, during the third dose. Of these six dogs, one male and one female were also observed salivating during the second IV administration of cosyntropin injection. One of the 5X, IV-dosed dogs had a hypersensitivity reaction to its third injection. The reaction started within 3 minutes of dosing and included: transient salivation, injected mucous membranes, inguinal erythema, facial edema, and tachycardia. After 1 hour, the facial edema had improved; the other signs persisted for approximately 150 minutes. The dog recovered without medical intervention. One of four 1X, IV-dosed dogs and three of eight 5X, IV-dosed dogs vomited once within an hour of dosing.
- STORAGE INFORMATION
-
HOW SUPPLIED
Cosacthen® (cosyntropin injection) is supplied in a clear glass vial with 1 mL cosyntropin (0.25 mg/mL).
NDC: 17033-390-01
Approved by FDA under NADA # 141-576

- SPL UNCLASSIFIED SECTION
-
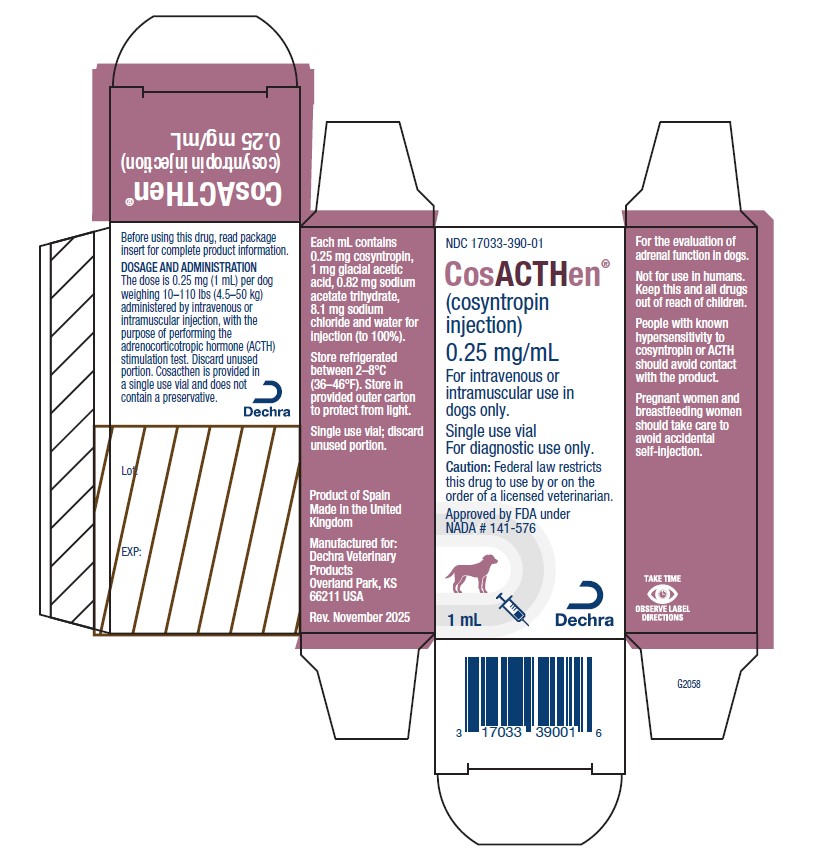
PRINCIPAL DISPLAY PANEL - 1 mL Vial
NDC: 17033-390-01
CosACTHen®
(cosyntropin injection)0.25 mg/mL
For intravenous or intramuscular use in dogs only.
Single use vial
For diagnostic use only.Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-576
1 mL
Dechra
-
INGREDIENTS AND APPEARANCE
COSACTHEN
cosyntropin injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17033-390 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COSYNTROPIN (UNII: 72YY86EA29) (COSYNTROPIN - UNII:72YY86EA29) COSYNTROPIN 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17033-390-01 1 in 1 CARTON 1 1 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141576 01/31/2026 Labeler - Dechra Veterinary Products, LLC (362142734)
Trademark Results [CosACTHen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COSACTHEN 97747465 not registered Live/Pending |
Dechra Limited 2023-01-09 |
 COSACTHEN 85931902 not registered Dead/Abandoned |
Novartis AG 2013-05-14 |
 COSACTHEN 79190584 5121625 Live/Registered |
Dechra Limited 2016-06-06 |
 COSACTHEN 77803817 not registered Dead/Abandoned |
Novartis AG 2009-08-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
