PSORIASIS CONTROL by Genuine Virgin Aloe Corporation
PSORIASIS CONTROL by
Drug Labeling and Warnings
PSORIASIS CONTROL by is a Otc medication manufactured, distributed, or labeled by Genuine Virgin Aloe Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
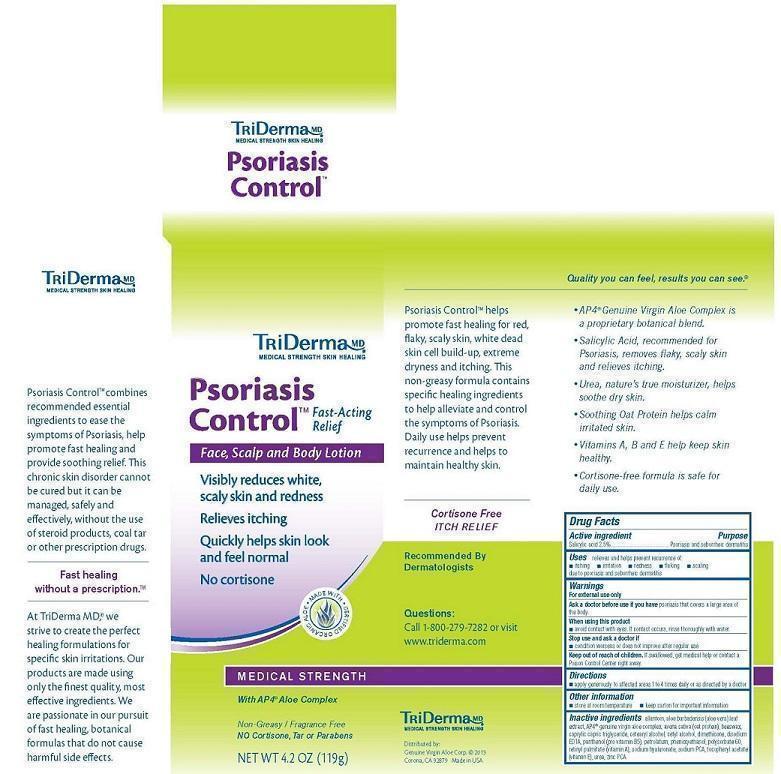
PSORIASIS CONTROL- salicylic acid cream
Genuine Virgin Aloe Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses:
relieves and helps prevent recurrence of:
- itching
- irritation
- redness
- flaking
- scaling
due to psoriasis and seborrheic dermatitis
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
Inactive ingredients:
allantoin, aloe barbadensis (AP4® genuine virgin organic aloe vera) leaf extract, avena sativa (oat protein), beeswax, caprylic capric triglyceride, cetearyl alcohol, cetyl alcohol, dimethicone, disodium EDTA, panthenol (pro vitamin B5), petrolatum, phenoxyethanol, polysorbate 60, retinyl palmitate (vitamin A), sodium hyaluronate, sodium PCA, tocopherol acetate (vitamin E), urea, zinc PCA
| PSORIASIS CONTROL
salicylic acid cream |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Genuine Virgin Aloe Corporation (961374147) |
| Registrant - Genuine Virgin Aloe Corporation (961374147) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genuine Virgin Aloe Corporation | 961374147 | manufacture(10738-101) | |
Trademark Results [PSORIASIS CONTROL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PSORIASIS CONTROL 86337840 4673292 Live/Registered |
GENUINE VIRGIN ALOE CORPORATION 2014-07-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.